Antiseptic active cationic dye and its preparing method
A reactive dye and cationic technology, which is applied in the field of antibacterial cationic reactive dye and its preparation, can solve the problems of lack of affinity for cotton fiber, loss of antibacterial properties of dyes, easy hydrolysis and other problems, and achieve excellent antibacterial durability and excellent dyeing affinity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
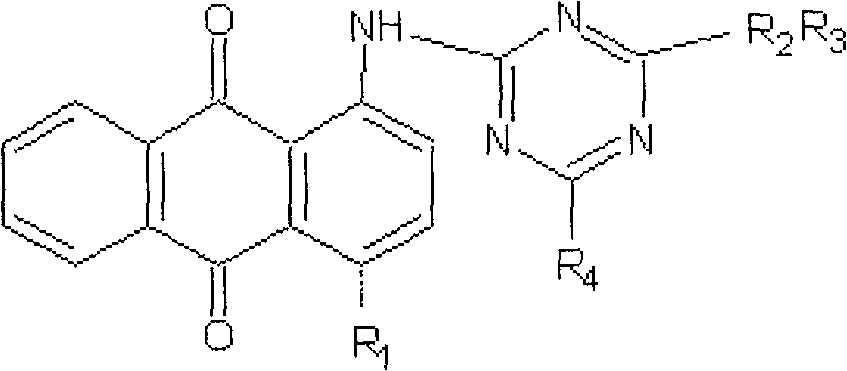
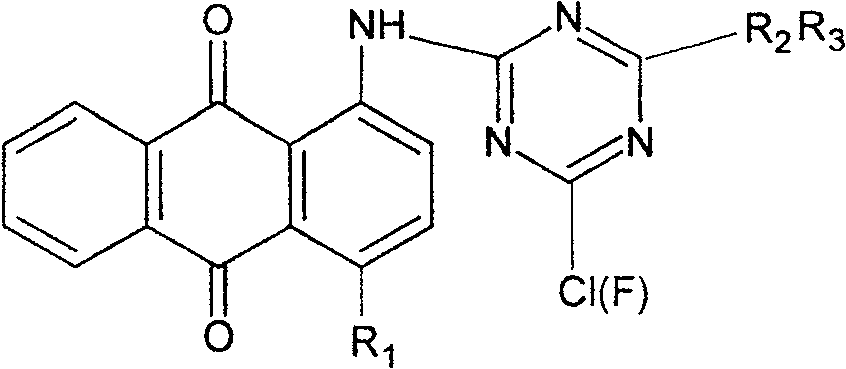
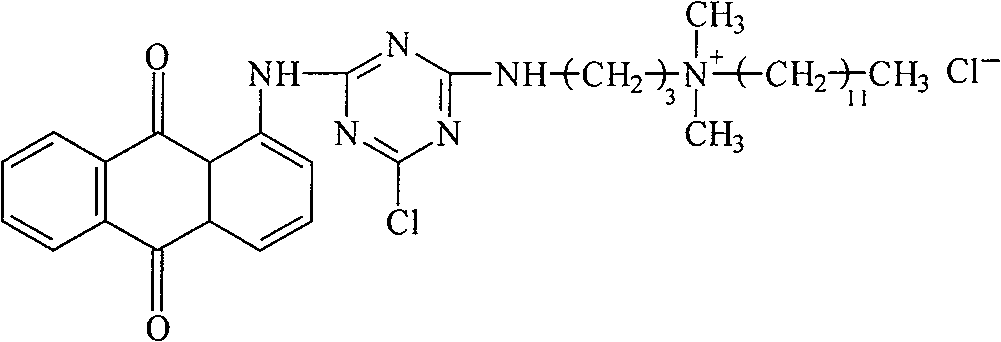
[0018] a. Dissolve 17g of 1-aminoanthraquinone and 20g of trifluoro-s-triazine in 200ml of nitrobenzene organic solvent, heat the mixture to 90°C, and raise the temperature to 120°C after 1 hour, at this temperature Continue to stir for about 1 hour, cool to 25-30°C, filter to obtain the dye intermediate 1-(4,6-dichloro-2-amino-s-triazine)aminoanthraquinone, wash with methanol, and dry in a vacuum dryer;
[0019] b. the dye intermediate 1-(4,6-dichloro-2-amino-s-triazine) aminoanthraquinone 10g prepared in step a is dissolved in 150ml DMF (N,N-dimethylformamide) organic solvent, heated to 55°C, slowly drop 5.5 g of 1-amino-3-dimethylaminopropane in 30 minutes, and then react for 2 hours. After the reaction is complete, the solvent DMF is evaporated in a vacuum evaporator to obtain the dye intermediate 1-( 4-chloro-6-(1-amino-3-dimethylaminopropane)-2-amino-s-triazine) aminoanthraquinone, dried in a vacuum dryer;
[0020] c. Dissolve 9 g of the dye intermediate prepared in ste...
Embodiment 2-21
[0024] Example 2-21, the synthesis method of the antibacterial cationic reactive dye is basically the same as that of Example 1, and see Table 1 for other conditions.
[0025] Table 1 Examples 2-21
[0026]
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com