Process of producing anti-humen CD20 monoclone antibody by animal mammary gland
A monoclonal antibody, human-mouse chimeric antibody technology, applied in the field of genetic engineering, can solve the problems of high cost, low yield of cell-expressed antibody, complicated purification process, etc., and achieve the effect of reduced production cost, huge economic benefits and social effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
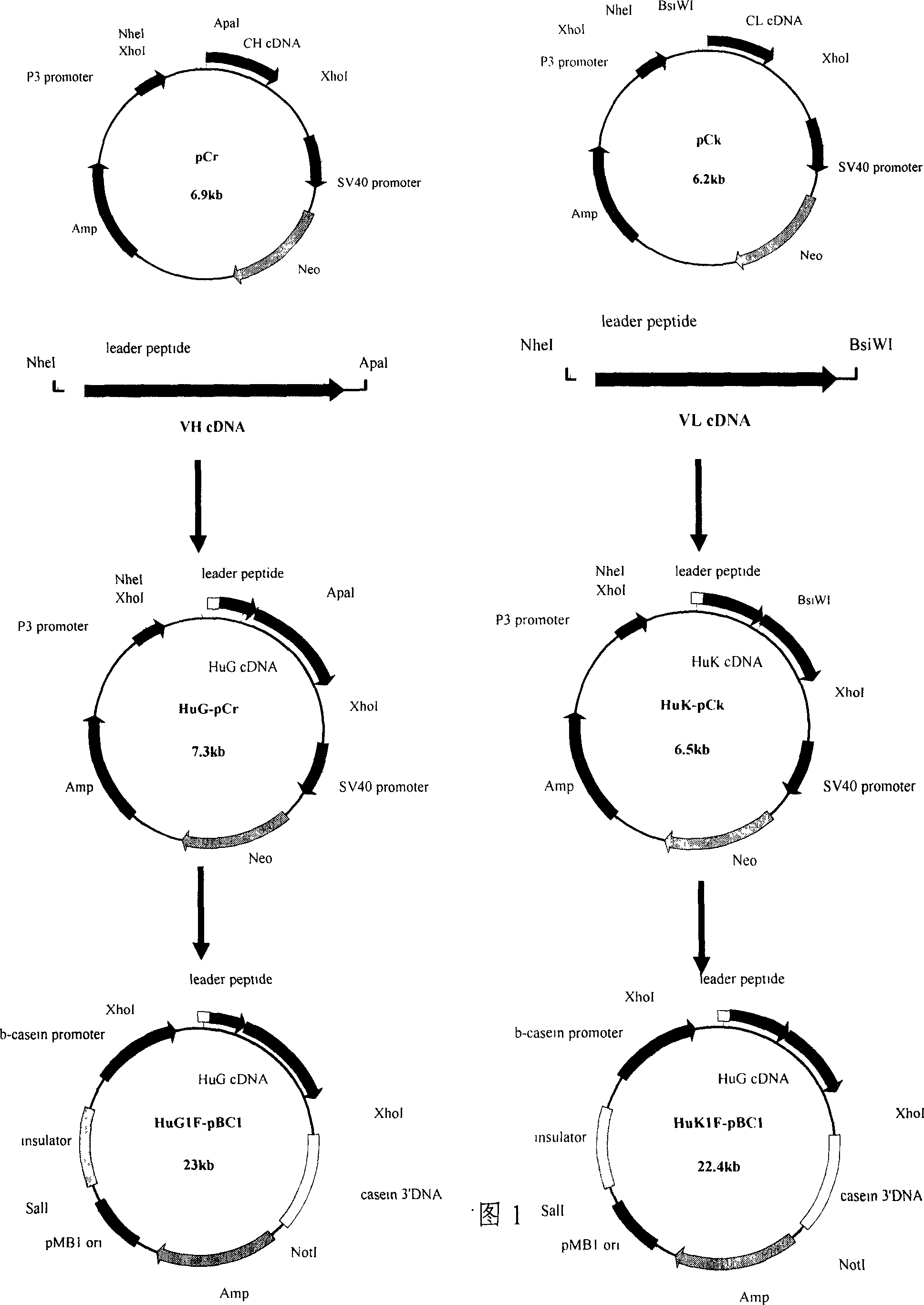
[0048] Embodiment 1 Construction of mammary gland expression vector of the present invention
[0049] 1.1 Construction of heavy chain mammary gland expression vector of anti-CD20 human-mouse chimeric antibody
[0050] According to Kabat EA et al. (Sequences of Proteins of Immunological Interest (ed5). NIH Publication No. 91-3242, 1991) announced the mouse anti-human CD20 monoclonal antibody heavy chain variable region sequence and its leader peptide sequence, synthetic mouse The heavy chain variable region sequence of anti-human CD20 monoclonal antibody (completed by Shanghai Sangong).
[0051] Because the human immunoglobulin gamma heavy chain constant region in the pCγ vector contains restriction sites such as XhoI, NheI, and ApaI at the 5' end, and XhoI at the 3' end, and the foreign gene insertion site of the mammary gland expression vector pBC1 is XhoI. In order to insert the synthetic heavy chain variable region sequence into the pCγ vector containing the human immunogl...
Embodiment 2
[0057] Example 2 Establishment and detection of a transgenic mouse model expressing anti-human CD20 monoclonal antibody 2.1 Microcoinjection of double genes to produce transgenic mice
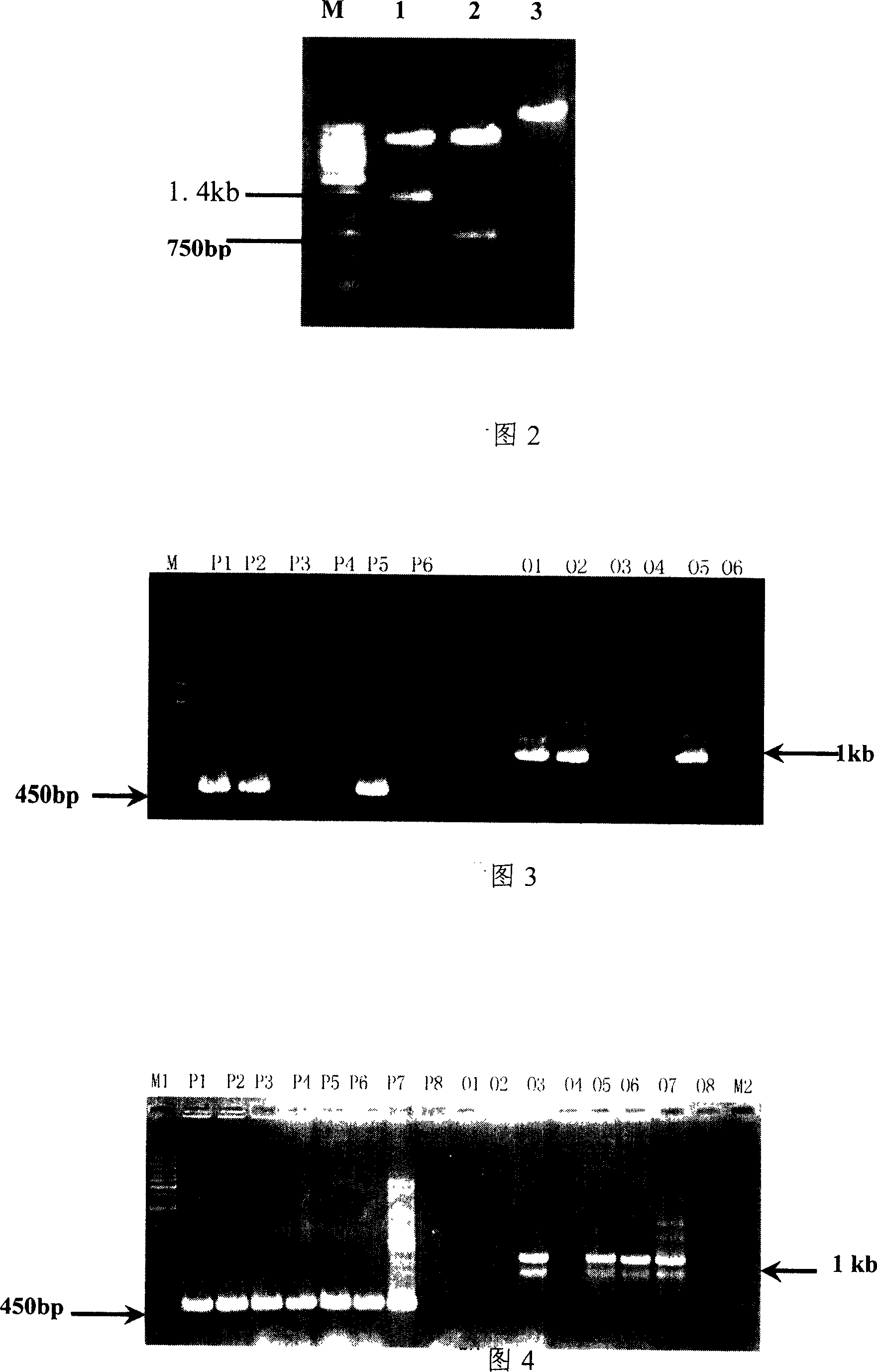
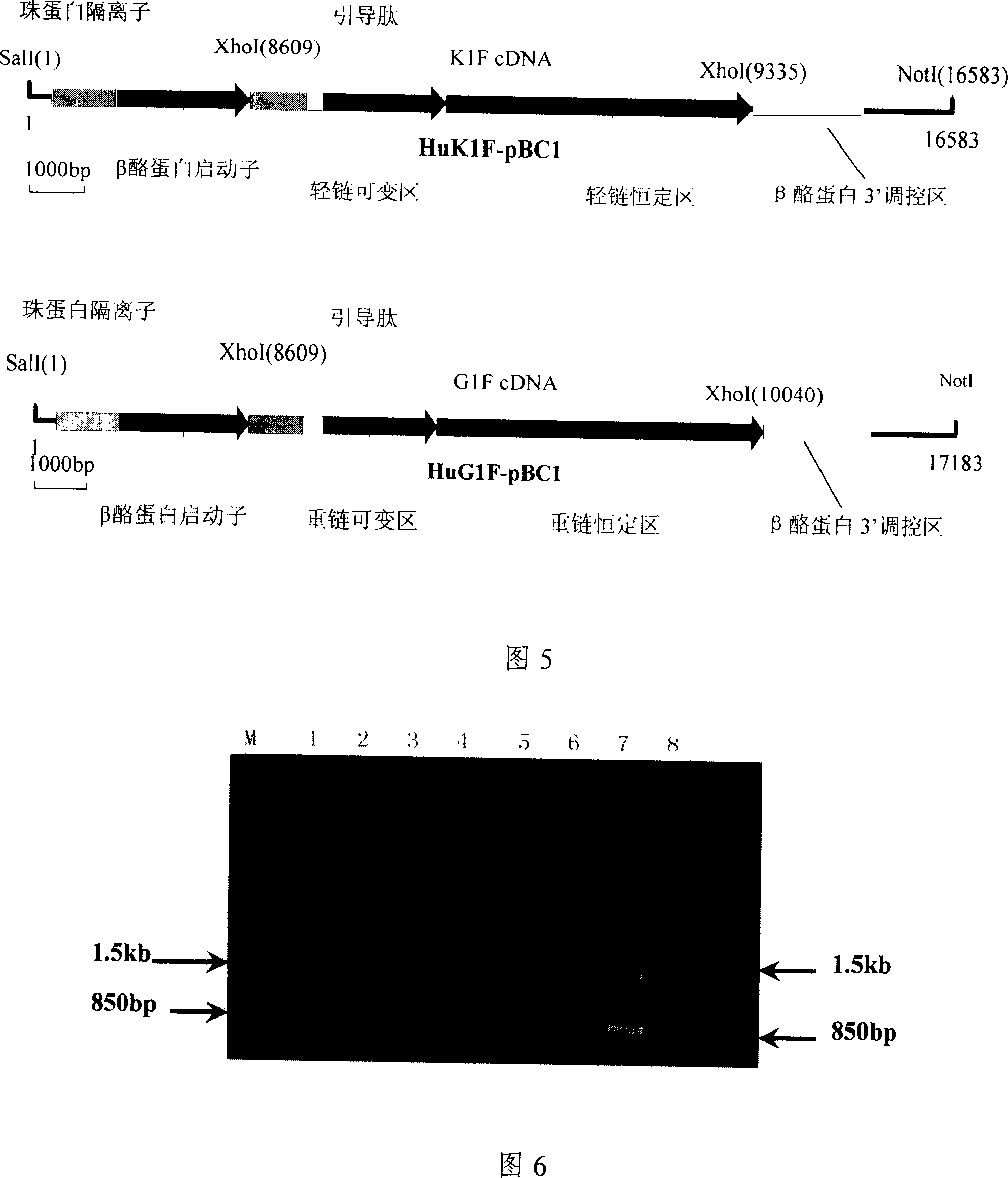
[0058] Production of transgenic mice by microinjection has a higher efficiency of linear integration than circular integration, so the constructed expression vector must first be enzymatically cut into a linear shape before microinjection. The light chain expression vector pBC1-HuK1F and the heavy chain expression vector pBC1-HuG1F were digested with NotI and SalI, the prokaryotic vector part was removed, and the 16.5kb exogenous gene fragment containing the light chain of the chimeric antibody and the 17.2kb fragment containing the heavy chain of the chimeric antibody were recovered. Chain exogenous gene fragments, the structure of the recovered fragments is shown in Figure 5. The recovery of exogenous gene fragments adopts the method of electroelution recovery first, and then the method of se...
Embodiment 3
[0068] Example 3 Preparation and detection of transgenic cattle expressing anti-human CD20 monoclonal antibody
[0069] 3.1 Preparation of transgenic cloned cattle
[0070] Fetal ear tissues of 5-month-old Holstein cows were taken, and the bovine fetal fibroblast cell line was established through primary culture, subculture, freezing and other in vitro culture operations.
[0071] The light chain mammary gland expression vector pBC1-HuK1F, the heavy chain mammary gland expression vector pBC1-HuG1F, and the double-marker selection vector pCE-EGFP-IRES-NEO (see Chinese invention patent CN03160031X) were respectively digested with SalI, and the light chains containing the chimeric antibody were respectively recovered. The gene fragment containing the heavy chain of the chimeric antibody, and the gene fragment containing the two selection markers of green fluorescent protein and neomycin resistance, the recovered gene fragments were dissolved in TE solution, and then the recovered...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com