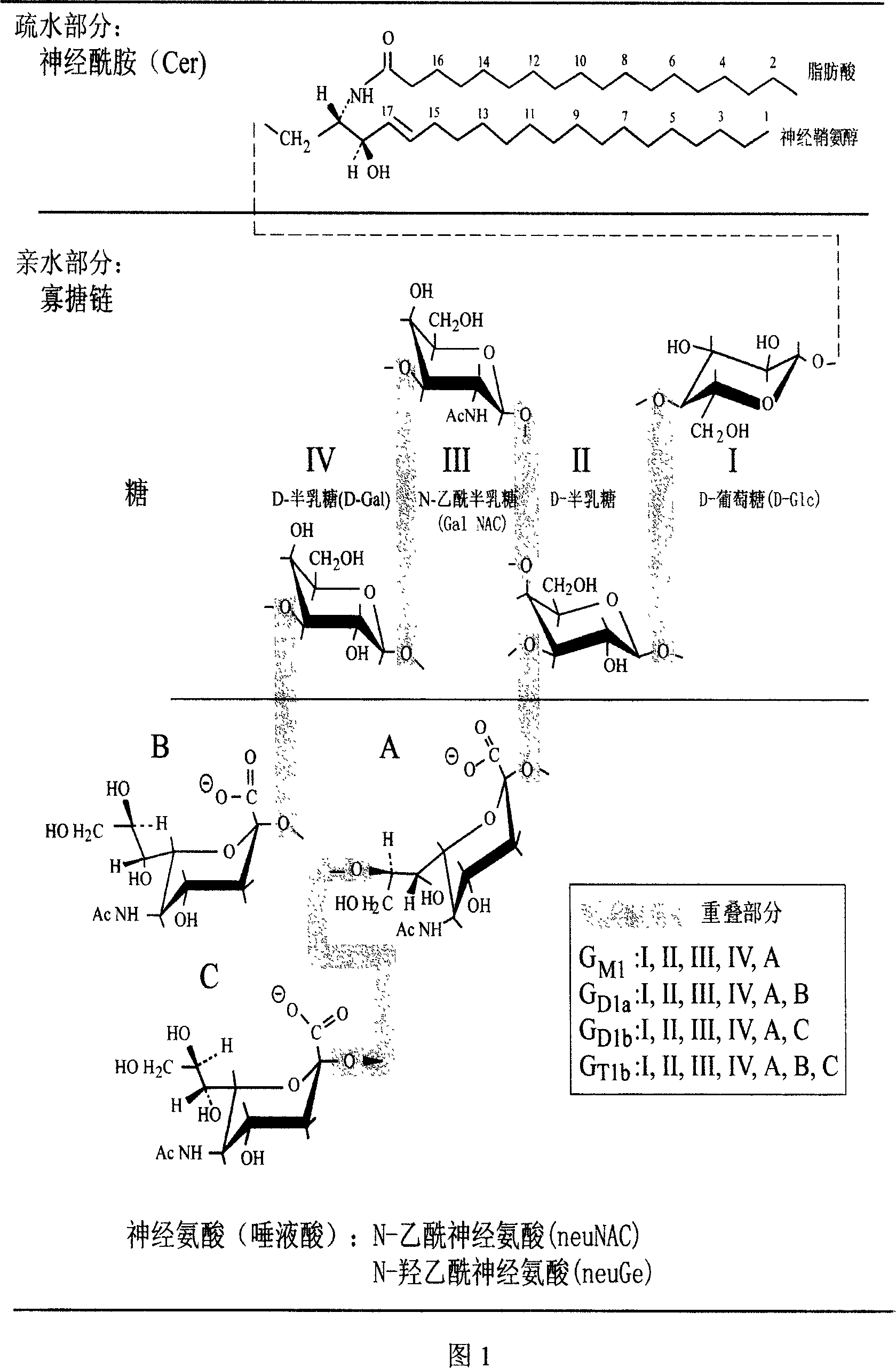
Method for extracting ganglioside with biological activity from animal tissues
A technology of gangliosides and animal tissues, applied in sugar derivatives, sugar derivatives, preparation of sugar derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Wash 100 grams of pig brain in ice water, rinse it with deionized water, stir it into a slurry with a pulverizer, add 200ml of acetone pre-cooled to 4°C for extraction, filter it with a Buchner funnel, and use the same amount of acetone to filter the cake Process once and drain. Add 50ml of sodium dihydrogen phosphate-disodium hydrogen phosphate buffer solution to the obtained filter cake, stir well, add 200ml of cold tetrahydrofuran for extraction, and suction filter with a Buchner funnel. The residue is treated once in the same way. The filtrates were combined and placed in a refrigerator at -17°C to freeze to precipitate an ice crystal solid (A), and the liquid phase (B) was separated and removed. (A) was distilled under reduced pressure at 35°C, concentrated to 80ml, added 400ml of cold acetone, stood overnight in the refrigerator, filtered to obtain a white solid, and dried in vacuum at -17°C to obtain 7-9g of crude product.
Embodiment 2
[0022] Wash 4 pig brains in ice water, rinse them with deionized water, grind them into a slurry with a pulverizer, add 250ml of acetone pre-cooled to -5°C for extraction, and filter twice (400ml acetone). Add 50 ml of citric acid-disodium hydrogen phosphate buffer solution to the residue, stir well, add 250 ml of cold tetrahydrofuran for extraction, filter, and repeat this process once to obtain 600 ml of filtrate. The filtrate was frozen in a -20°C refrigerator until all ice crystals were precipitated, and the ice cubes were taken out and distilled under reduced pressure at 30°C to concentrate from about 400ml to about 80ml. Add 320ml of cold acetone at 1°C to precipitate a solid, separate the solid, and dry it under vacuum at -17°C to obtain a white solid product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com