Extract of an traditional Chinese medicine containing isopsoralen, preparation method and use thereof
A technology of isopsoralen and extract, which is applied in the field of traditional Chinese medicine extract, achieves the effect of simple preparation, obvious inhibitory effect, and quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 Preparation of psoraleae extract of the present invention
[0019] Weigh 500 g of psoraleae medicinal material, dissolve it in 2 L of pure water, heat and reflux for 5 hours, use a Soxhlet extraction device to collect volatile oil components, and obtain a volatile oil extract. Add 2L of industrial alcohol (95% ethanol) to the obtained dregs, extract in a water bath at 99° C. for 2 hours under reflux to obtain extract 1, and concentrate extract 1 to obtain about 100 g of extract. ODS-C18 column is used to separate it, and the sample extract is about 50g, and the flow rate is 10ml / min; first use 5% methanol as the mobile phase, then change 15% methanol as the mobile phase, then change 30% methanol as the mobile phase, and then Replace 50% methanol as the mobile phase, and then replace 70% methanol as the mobile phase to obtain eluent 1. The eluent 1 was concentrated and dried to obtain 1.55 g of the extract (psoralen extract).
Embodiment 2
[0020] Example 2 Composition analysis of psoraleae extract in the present invention
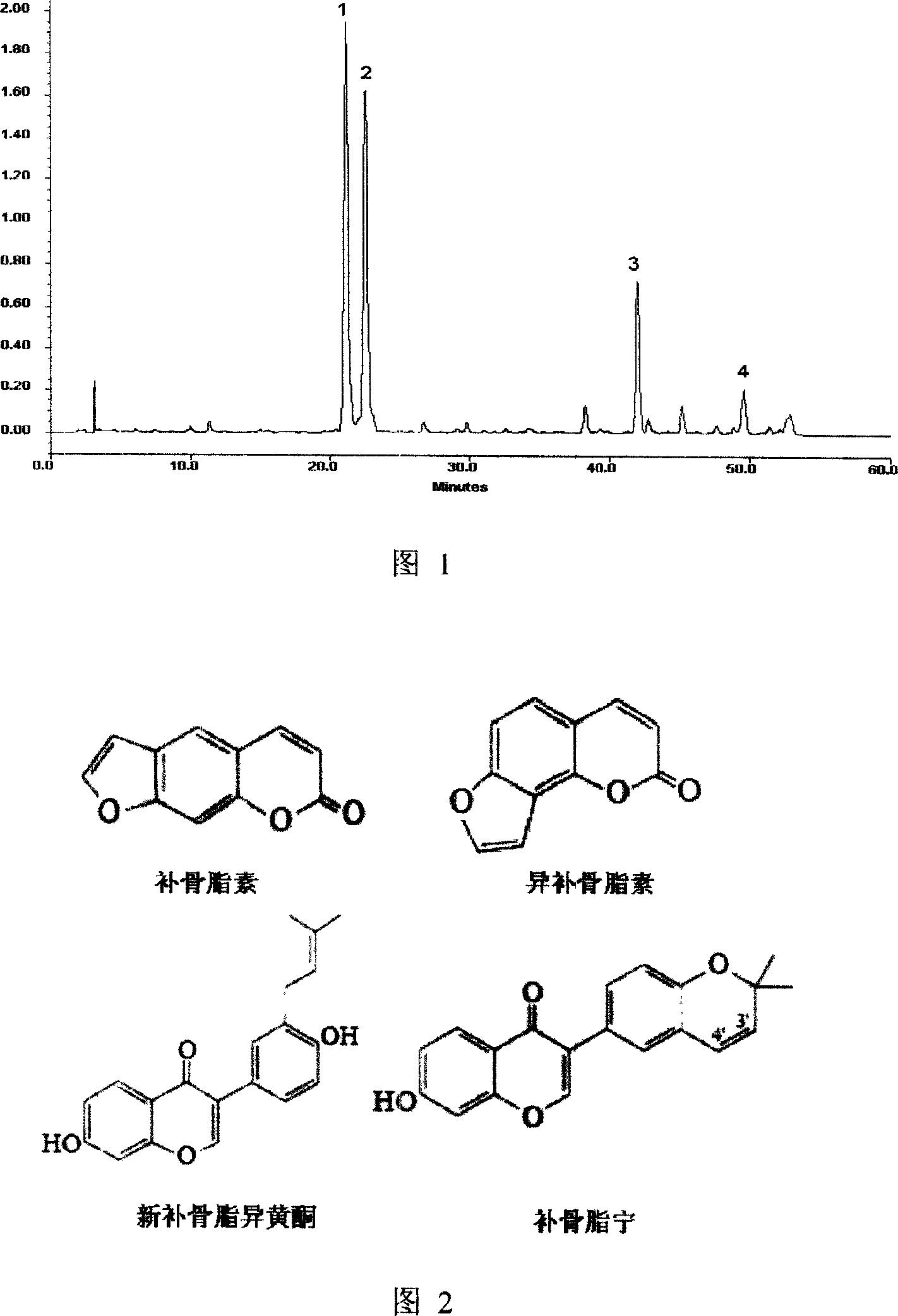
[0021] 1. Component analysis in psoraleae extract (high performance liquid chromatography)
[0022] Chromatographic conditions Chromatographic column: Agilent Zorbax SB-C18 chromatographic column (4.6mm×250mm, 5μm); column temperature: 30°C; mobile phase: (A) water, (B) acetonitrile, linear gradient elution, gradient elution program See Table 1; flow rate: 0.8mL min -1 ; Detection wavelength 190 ~ 400nm; analysis time 60min; injection volume: 10μL.
[0023] Table 1 Changes in mobile phase ratios for linear gradient elution
[0024] time
(min)
0
25
50
60
water%
75
25
65
35
50
50
30
70
[0025] Preparation of the test solution Weigh this product, dissolve it in a volumetric flask with methanol, dilute to the mark, shake well, and you get it.
[0026] Determina...
Embodiment 3
[0031] Example 3 Determination of the content of active ingredients in the psoralen extract of the present invention
[0032] 1. Determination of psoralen, isopsoralen, neopsoralen isoflavones and psoralenin in psoralen extract (high performance liquid chromatography)
[0033] For chromatographic conditions, refer to the first step of Example 2, 1. Component Analysis in Psoraleae Extract (High Performance Liquid Chromatography). The recorded chromatographic time was 55 minutes.
[0034] Preparation of the test solution Weigh psoralen, isopsoralen, new psoralen isoflavones and psoralenin standard and psoralen extract samples, dissolve them in a volumetric flask with methanol, and dilute to Scale, shake well, that is.
[0035] 2. Determination method Precisely absorb the test solution, inject it into a high performance liquid chromatograph, measure the peak area absorbed at 300nm, and calculate the content of the sample by the external standard method.
[0036] Analysis Resul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com