Compound 3,4-diaryl pyrazole and its prepn and medicinal use
A compound, pyrazole technology, applied in drug combination, organic chemistry, cardiovascular system diseases, etc., can solve problems such as weak effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
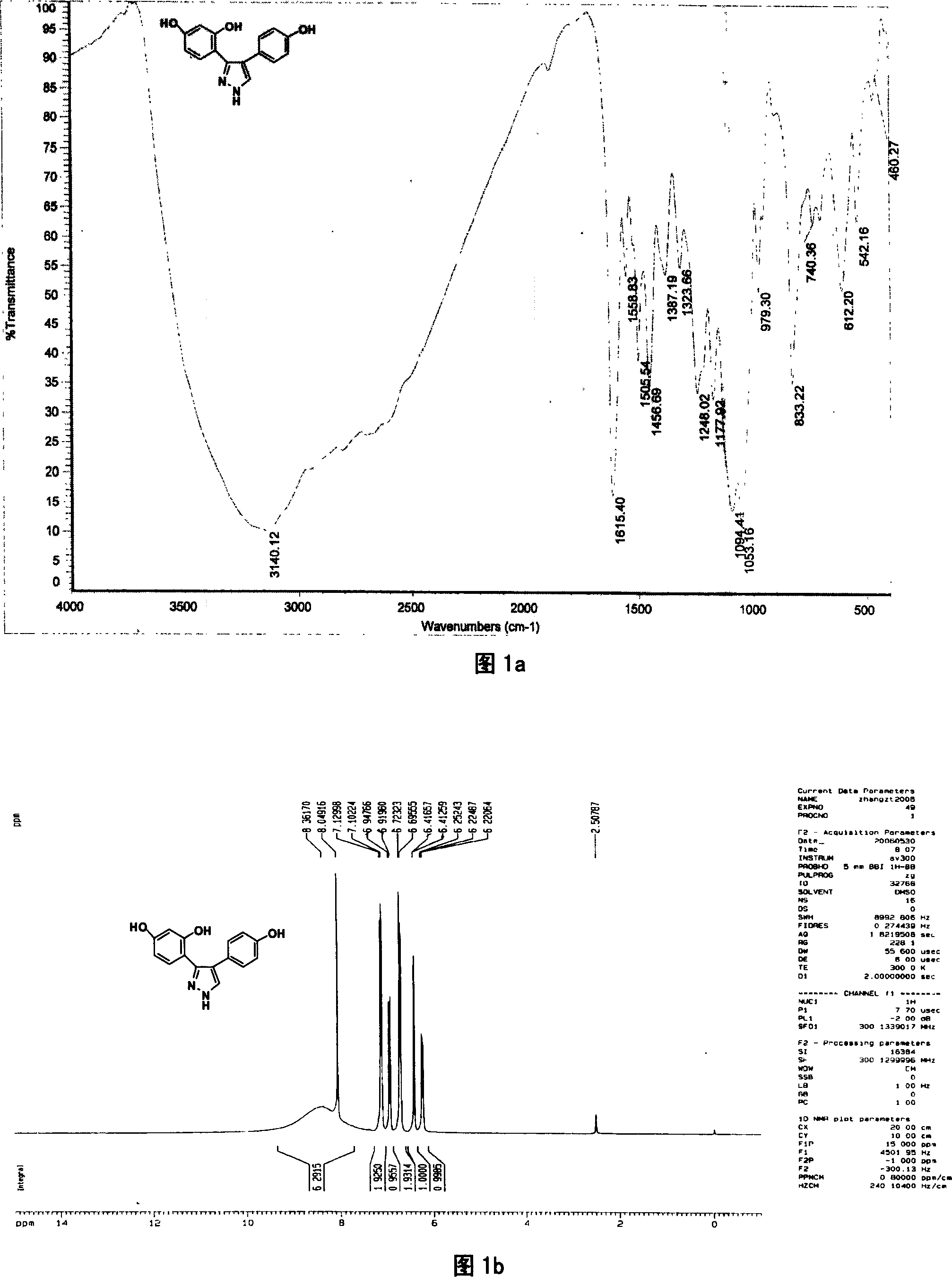
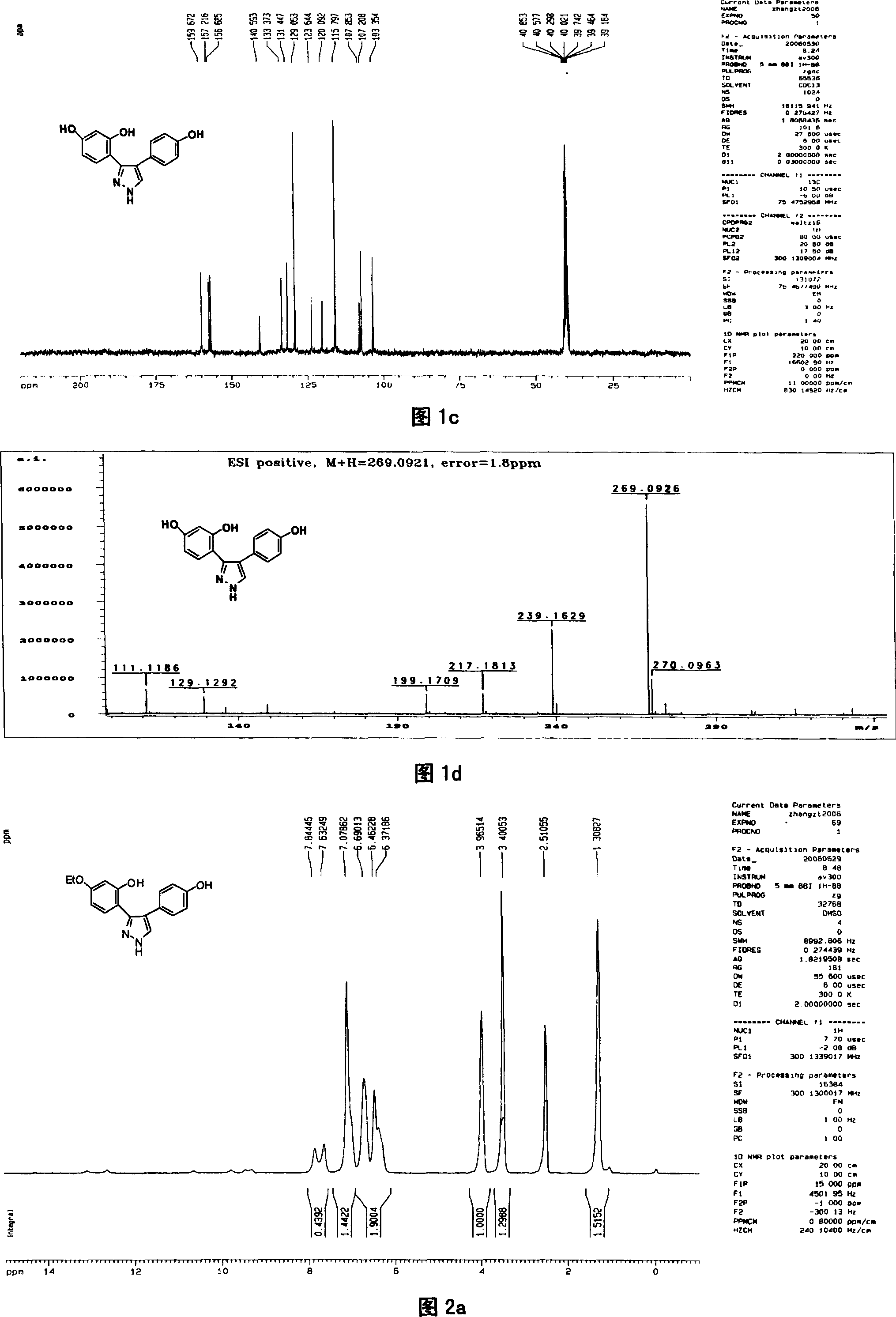
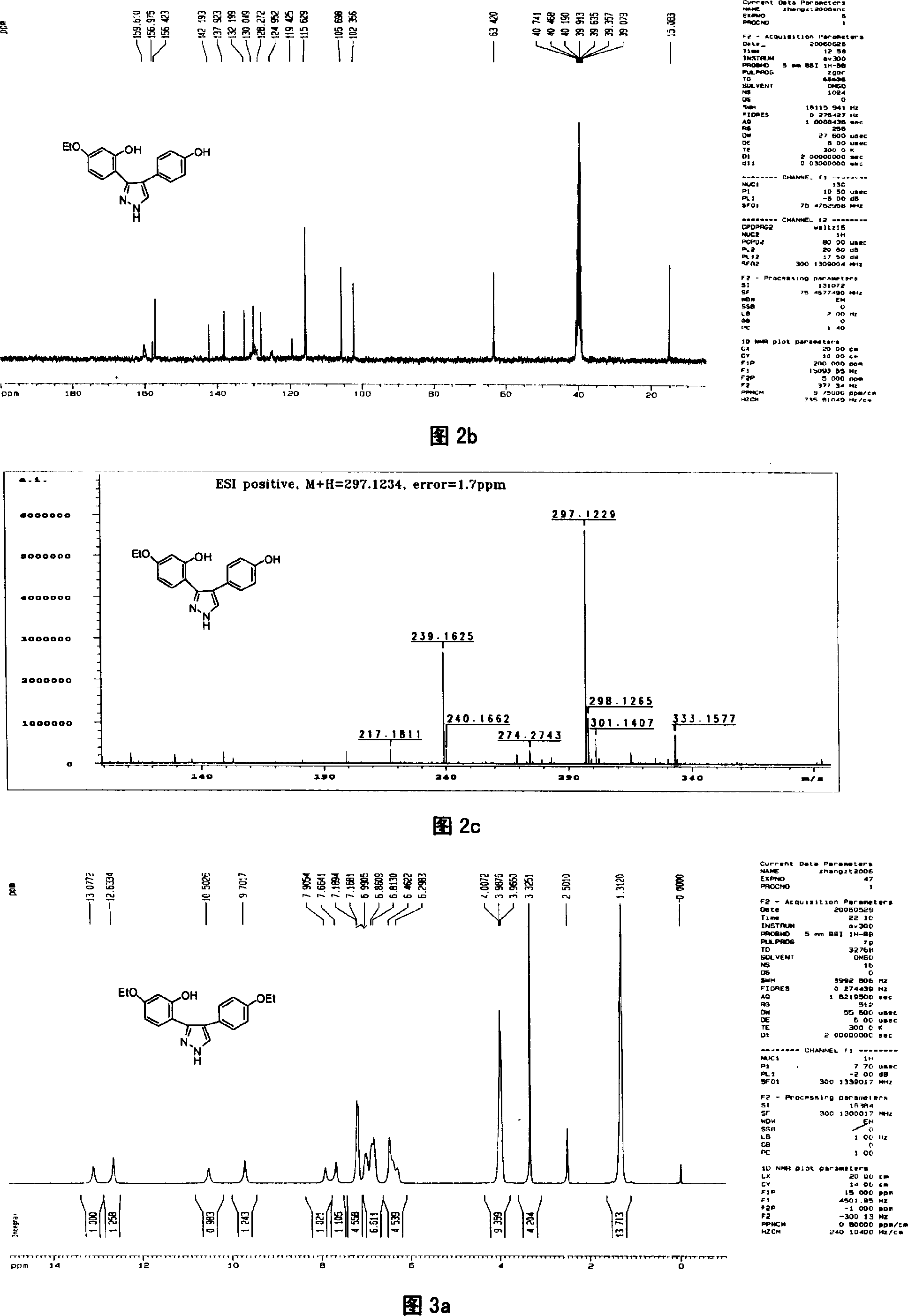
[0041] Preparation of compound 1 3-(2,4-dihydroxyphenyl)-4-(4-hydroxyphenyl)pyrazole
[0042] In this example, first add 4', 7-dihydroxy isoflavone to the reaction kettle, add 8-12 times the weight of 4', 7-dihydroxy isoflavone compound under stirring with a stirrer, and then add 80% hydration Hydrazine, 4', the molar ratio of 7-dihydroxy isoflavone and 80% hydrazine hydrate is 1: 5, after stirring with a stirrer, the temperature of the reaction solution is 80° C. with a temperature regulating device, reacted for 2.5 hours, and obtained 3-(2 , the mixture of 4-dihydroxyphenyl)-4-(4-hydroxyphenyl)pyrazole and unreacted raw materials, the mixture after the reaction is distilled under reduced pressure, then add 4 times the amount of distilled water of the total volume of the reactant, fully stir, static Leave overnight to form a precipitate, which is separated by suction filtration under reduced pressure and washed with distilled water until neutral to obtain a crude product of 3...
Embodiment 2
[0212] Preparation of compound 1 3-(2,4-dihydroxyphenyl)-4-(4-hydroxyphenyl)pyrazole
[0213] In this example, first add 4', 7-dihydroxy isoflavone to the reaction kettle, add 8-12 times the weight of 4', 7-dihydroxy isoflavone compound under stirring with a stirrer, and then add 80% hydration Hydrazine, 4', the molar ratio of 7-dihydroxy isoflavone and 80% hydrazine hydrate is 1: 10, after being stirred with a stirrer, the temperature of the reaction solution is made to be 80° C. with a temperature regulating device, and reacted for 2 hours to obtain 3-(2 , 4-dihydroxyphenyl)-4-(4-hydroxyphenyl)pyrazole and the mixture of unreacted raw materials, the mixture after the reaction is distilled under reduced pressure, add 4 times the amount of distilled water of the total volume of the reactant, fully stir, let stand Precipitate was generated overnight, separated by vacuum suction filtration and washed with distilled water until neutral. Other processes were the same as in Example...
Embodiment 3
[0217] Preparation of compound 1 3-(2,4-dihydroxyphenyl)-4-(4-hydroxyphenyl)pyrazole
[0218] In this example, first add 4', 7-dihydroxy isoflavone to the reaction kettle, add 8-12 times the weight of 4', 7-dihydroxy isoflavone compound under stirring with a stirrer, and then add 80% hydration Hydrazine, 4', the molar ratio of 7-dihydroxy isoflavone and 80% hydrazine hydrate is 1: 1, after stirring with a stirrer, the temperature of the reaction solution is made to be 60°C with a temperature regulating device, and react for 4 hours to obtain 3-(2 , the mixture of 4-dihydroxyphenyl)-4-(4-hydroxyphenyl)pyrazole and unreacted raw materials, the reaction mixture is distilled under reduced pressure, and distilled water is added to 1 times the total volume of the reactant, fully stirred, and left to stand Precipitate was generated overnight, separated by vacuum suction filtration and washed with distilled water until neutral. Other processes were the same as in Example 1.
[0219] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com