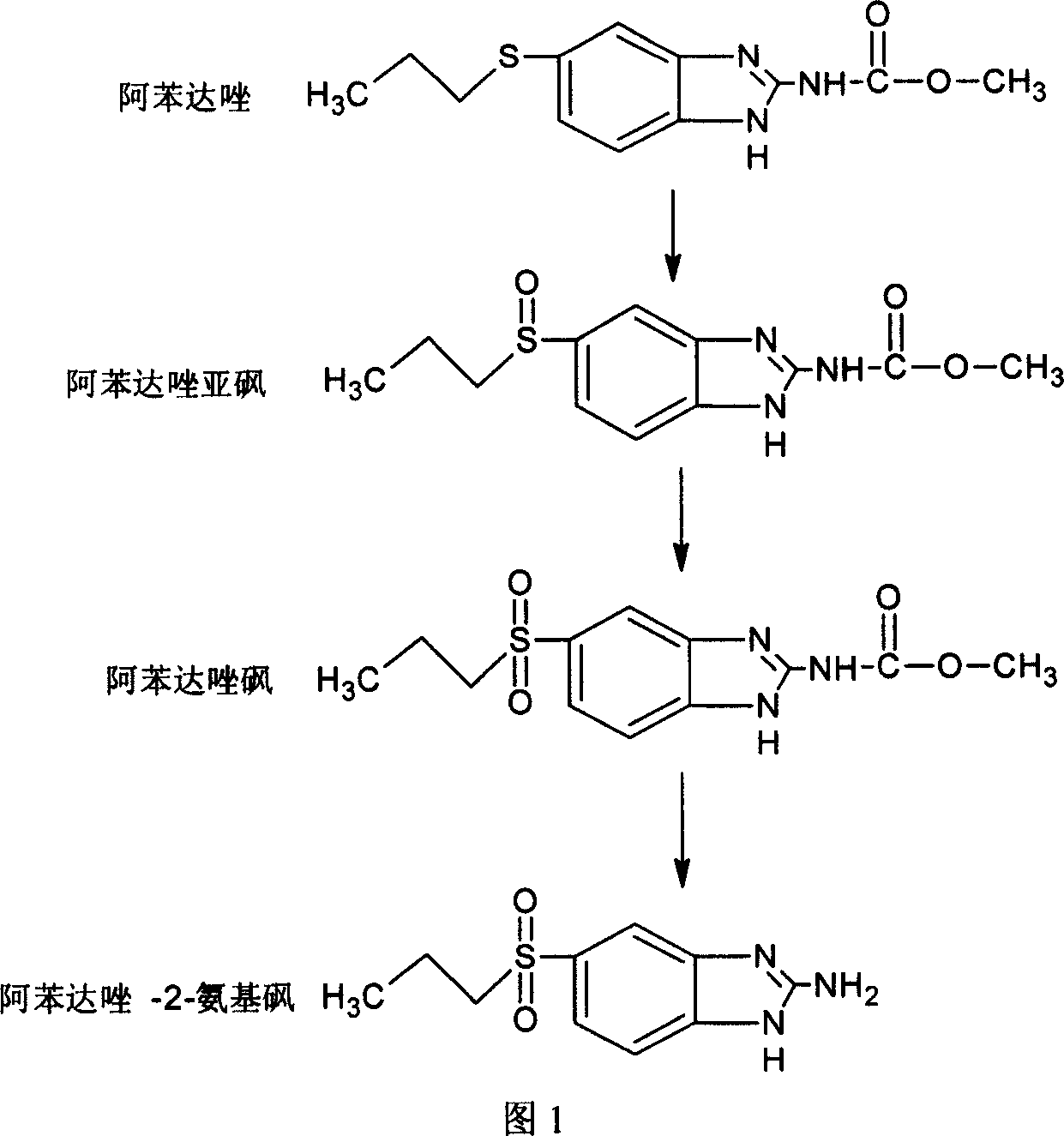
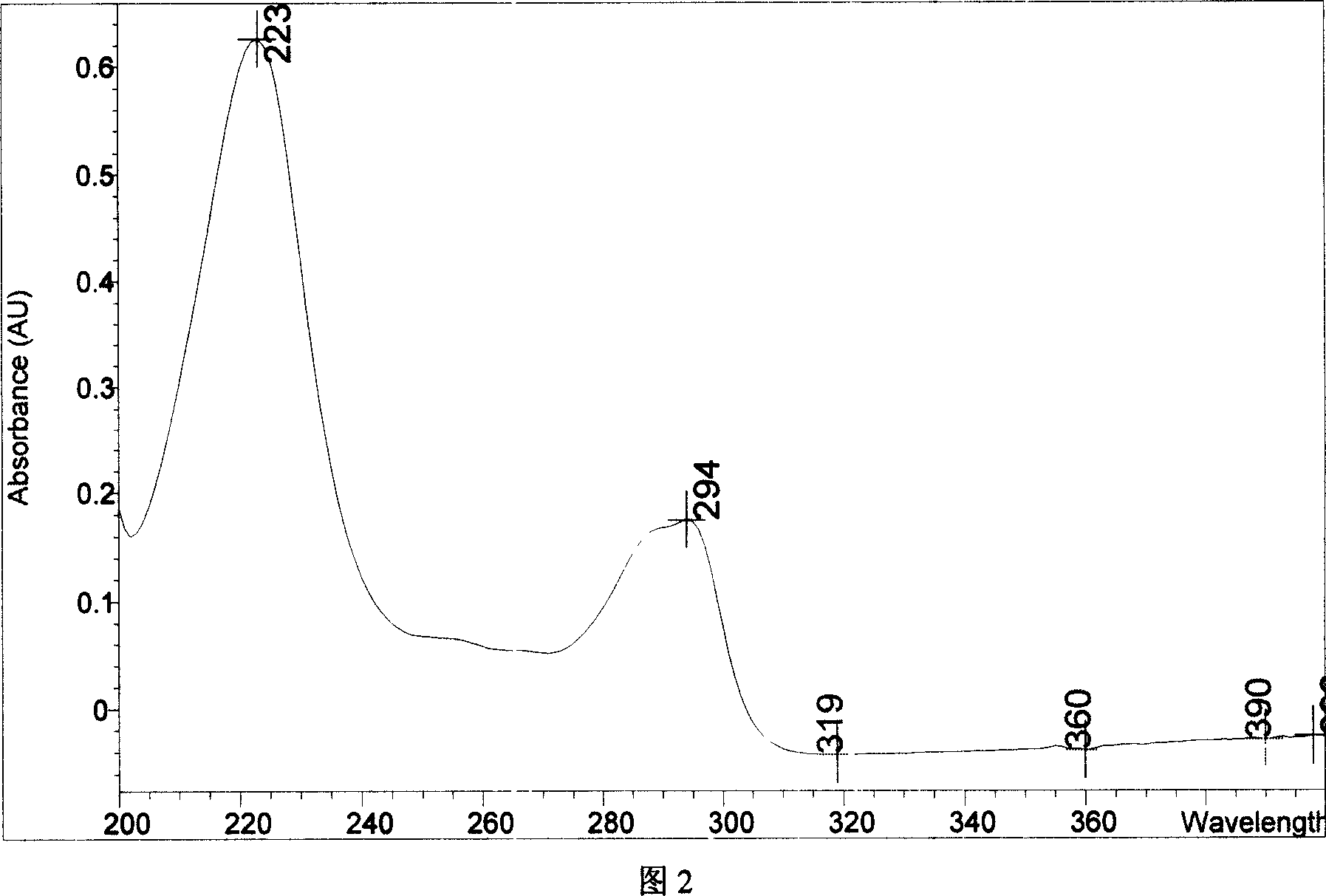
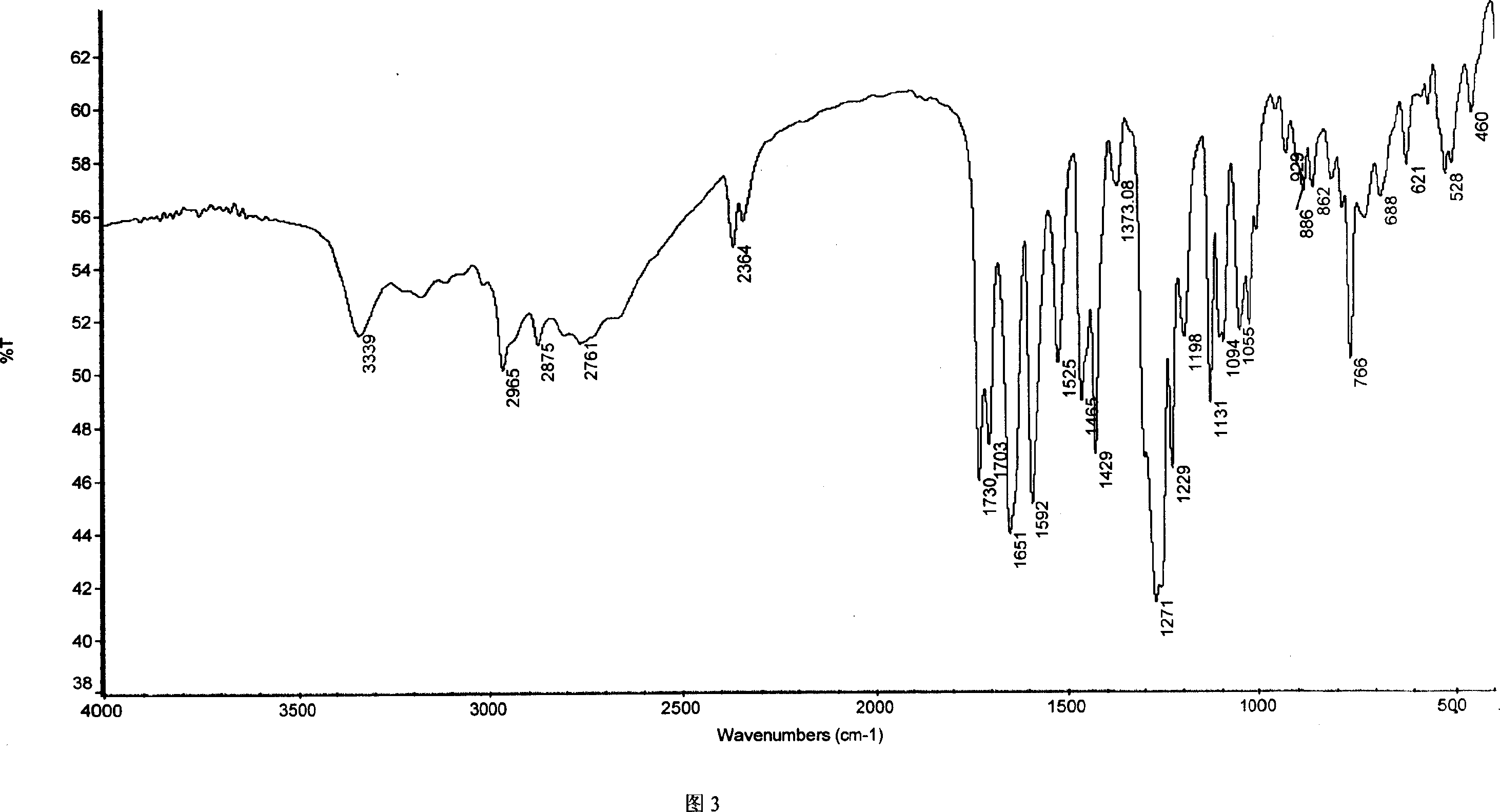
Chemical synthesis of albendazole-sulfoxide
A technology for the chemical synthesis of albendazole sulfoxide, applied in the direction of organic chemistry, which can solve the problems of complex purification methods, many reaction steps, and harsh conditions, and achieve the effect of simple purification methods, fewer reaction steps, and reduced production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 13.25g of albendazole into a 250mL four-neck flask, then add 70mL of glacial acetic acid, and stir at 15°C until dissolved. Slowly add 5.1 mL of 30% hydrogen peroxide dropwise with a dropping funnel, and react for 5 hours after the dropwise addition is completed. The end of the reaction is 1mol L -1 The reaction mixture was neutralized to pH 6.0 with a sodium hydroxide solution, filtered, and the filter cake was dried in an oven at 30°C. 13.76 g of crude albendazole sulfoxide was obtained.
[0026] Dissolve 10.02g of crude albendazole sulfoxide in a three-neck flask equipped with a spherical condenser, place in a 90°C water bath, add 100mL of 70% ethanol solution and stir until dissolved, suction filter while it is hot, slowly cool the filtrate to 4°C, and place it for 5~ Filter after 10h, the filter cake is rinsed with ethanol to obtain a recrystallization product of albendazole sulfoxide, and after the product is dried, the acetonitrile solution of N,N-dimethylf...
Embodiment 2
[0028] Add 13.25g of albendazole into a 250mL four-necked flask, add 70mL of glacial acetic acid, and stir in a 35°C water bath until dissolved. Slowly add 5.1 mL of 30% hydrogen peroxide dropwise with a dropping funnel, and react for 4 hours after the dropwise addition is completed. The reaction ends with 5mol L -1 The reaction mixture was neutralized to pH 6.5 with a sodium hydroxide solution, filtered, and the filter cake was dried in an oven at 40°C. 13.85 g of crude albendazole sulfoxide was obtained.
[0029] Dissolve 10.12g of crude albendazole sulfoxide in a three-neck flask equipped with a spherical condenser, place it in a water bath at 90°C, add 100mL of 85% ethanol solution and stir until dissolved, suction filter while it is hot, slowly cool the filtrate to 4°C, and place it for 5- After 10h, filter and wash the filter cake with ethanol to obtain a recrystallized product of albendazole sulfoxide. ) recrystallized twice, the filter cake was rinsed with N, N-dime...
Embodiment 3
[0031] Add 13.25g of albendazole into a 250mL four-necked flask, add 70mL of glacial acetic acid, and stir in a 50°C water bath until dissolved. Slowly add 5.1 mL of 30% hydrogen peroxide dropwise with a dropping funnel, and react for 3 hours after the dropwise addition is completed. The reaction ends with 8mol·L -1 The sodium hydroxide solution was neutralized to pH 7.0, filtered, and the filter cake was dried in an oven at 50°C. 13.71 g of crude albendazole sulfoxide was obtained.
[0032] Dissolve 10.07g of albendazole sulfoxide crude product in a three-neck flask equipped with a spherical condenser, place it in a water bath at 90°C, add 100mL of 90% ethanol solution and stir until dissolved, suction filter while it is hot, slowly cool the filtrate to 4°C, and place it for 5- After 10 hours, filter, and wash the filter cake with ethanol to obtain a recrystallized product of albendazole sulfoxide. After recrystallization twice, the filter cake was first washed with N,N-di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com