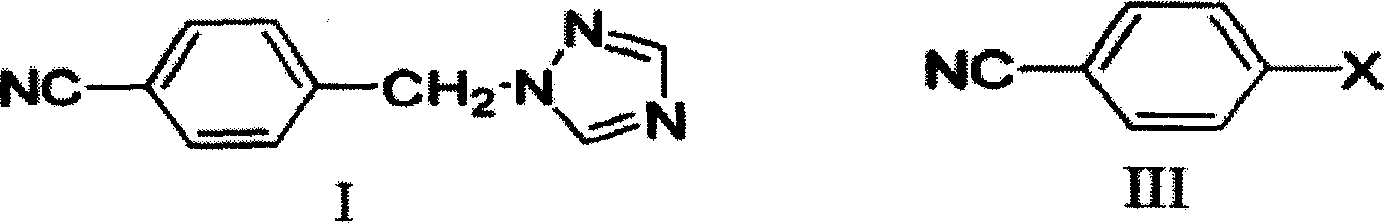
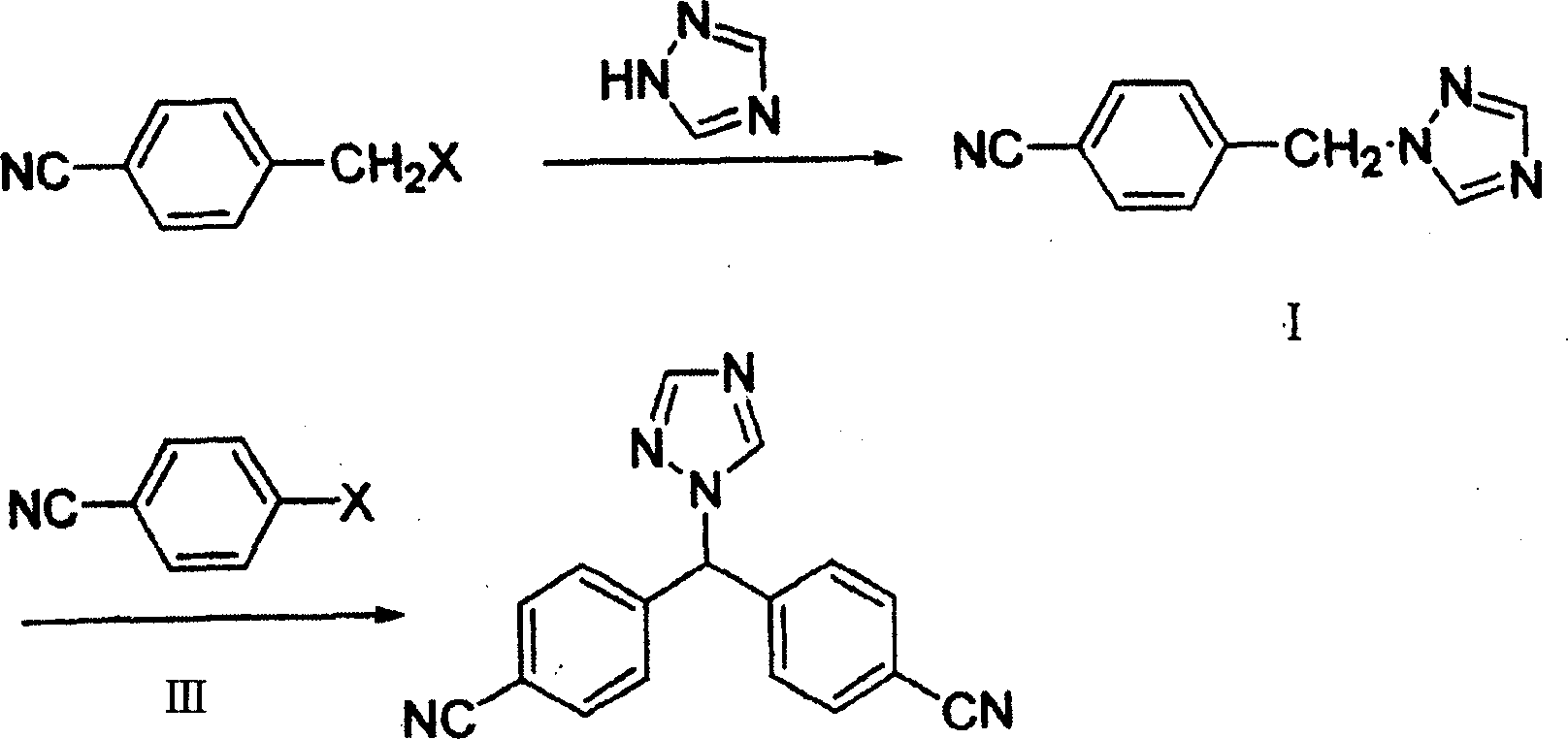
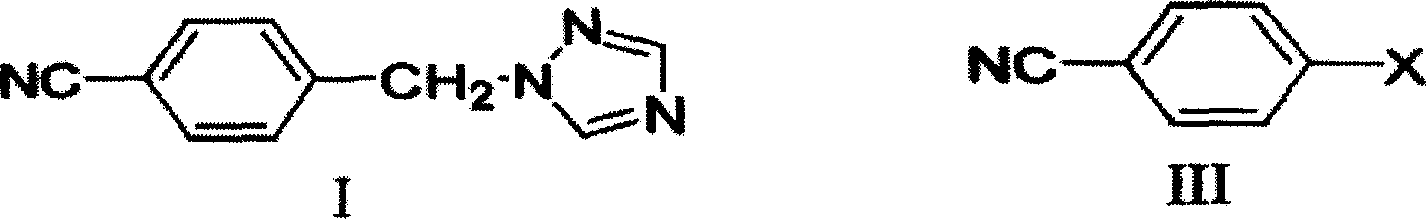
Method of preparing letrozole
A technology of letrozole and reaction, which is applied in the field of letrozole preparation, can solve problems such as great influence on yield, difficulty in obtaining potassium tert-butoxide, strong alkalinity of potassium tert-butoxide, etc., and achieve high production efficiency and low price , less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 100 ml of DMF to the reaction flask and dissolve 7.3 g of sodium ethoxide. After stirring, 100 ml of DMF solution containing 10 g of Compound I was slowly added, and the reaction temperature was controlled at 10°C. After the addition was complete, stirring was continued for one hour at room temperature. After stirring, 50 ml of a DMF solution containing 8 g of p-fluorobenzonitrile was slowly added at room temperature. Stirring was continued for half an hour after the addition was complete. The pH was adjusted to 7 with concentrated hydrochloric acid, and the solvent was removed under reduced pressure. An appropriate amount of water was added, extracted three times continuously with dichloromethane, and the organic phases were combined and washed with saturated brine. After drying over magnesium sulfate, the solvent was distilled off to obtain a crude product. Recrystallization with ethyl acetate gave 12.8 g of pure product with a yield of 82.7%. Product melting...
Embodiment 2
[0030] Add 100 ml of DMF to the reaction flask, and dissolve 12.1 g of potassium tert-butoxide. After stirring, 100 ml of DMF solution containing 10 g of Compound I was slowly added dropwise, and the reaction temperature was controlled at 10°C. After the addition was complete, stirring was continued for one hour at room temperature. After stirring, 50 ml of a DMF solution containing 8 g of p-fluorobenzonitrile was slowly added dropwise at room temperature. Stirring was continued for half an hour after the addition was complete. The pH was adjusted to 7 with concentrated hydrochloric acid, and the solvent was removed under reduced pressure. An appropriate amount of water was added, extracted three times continuously with dichloromethane, and the organic phases were combined and washed with saturated brine. After drying over magnesium sulfate, the solvent was distilled off to obtain a crude product. Recrystallization with ethyl acetate gave 7.8 g of pure product with a yield...
Embodiment 3
[0032]Add 100 ml of DMF to the reaction flask and dissolve 7.3 g of sodium ethoxide. After stirring, 100 ml of DMF solution containing 10 g of Compound I was slowly added, and the reaction temperature was controlled at 20°C. After the addition was complete, stirring was continued for one hour at room temperature. After stirring, 50 ml of a DMF solution containing 8 g of p-fluorobenzonitrile was slowly added at room temperature. Stirring was continued for half an hour after the addition was complete. The pH was adjusted to 7 with concentrated hydrochloric acid, and the solvent was removed under reduced pressure. An appropriate amount of water was added, extracted three times continuously with dichloromethane, and the organic phases were combined and washed with saturated brine. After drying over magnesium sulfate, the solvent was distilled off to obtain a crude product. Recrystallized from ethyl acetate to obtain 10.2 g of pure product with a yield of 66%. Product melting ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com