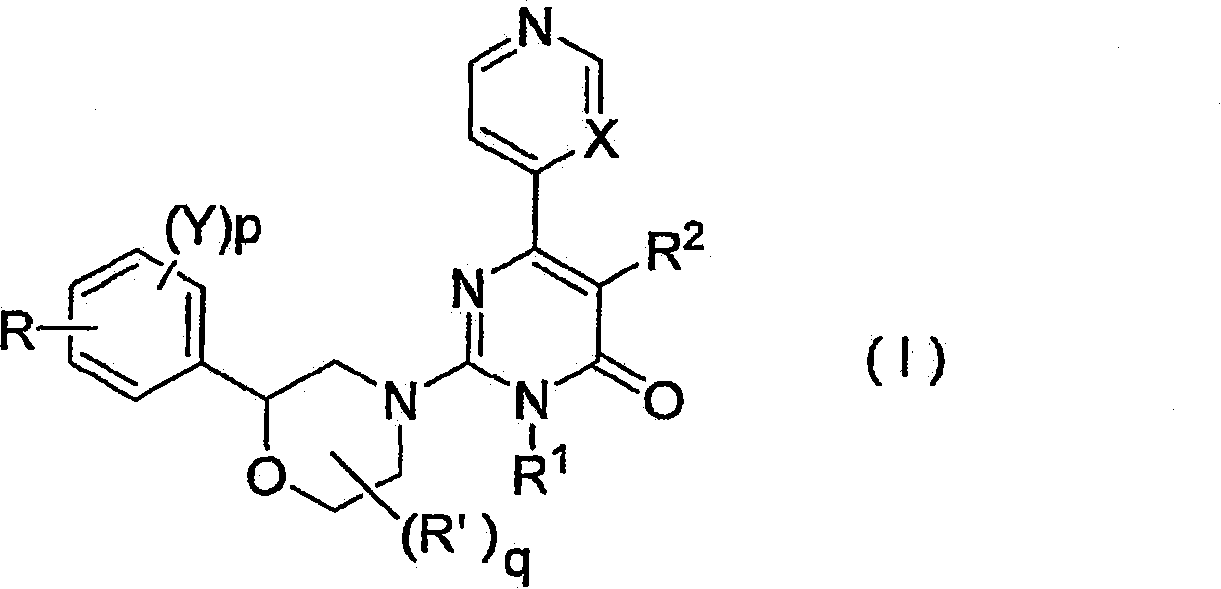
2-morpholino-4-pyrimidone compound
A compound and alkyl technology, applied in the field of 2-morpholino-4-pyrimidinone compounds, can solve the problems of insufficient use as medicines and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0529] N-(4-((2S)-4-(1-methyl-6-oxo-4-(pyridin-4-yl)-1,6-dihydropyrimidin-2-yl)-morpholine-2 -yl) phenyl) acetamide (compound number 39)
[0530] 2-Bromo-(1S)-1-(4-bromophenyl)ethanol
[0531] The borane-THF complex (1.0 M solution in THF, 270 mL, 270 mmol) was added to (S)-CBS ((S)-2-methanol) at -30 °C over 15 minutes base-CBS-oxazoboridine, 50 ml, 1.0 M toluene solution) solution and stirred for 15 minutes. 4-Bromophenacyl bromide (75.0 g, 270 mmol) in dichloromethane (350 mL) was then added dropwise over 70 minutes while maintaining the temperature at -32 to -28°C. After stirring for 1 hour, the resulting solution was warmed to room temperature, and methanol (10 mL) was added slowly, followed by dropwise addition of 0.5M hydrochloric acid (300 mL) over 10 minutes. After stirring for 40 minutes, the resulting solution was filtered and the filtrate was extracted with dichloromethane. The combined organic layers were washed with 0.5M hydrochloric acid, 0.1M aqueous sodium...
Embodiment 2
[0552]N-(4-((2S)-4-(1,6-dihydro-1-methyl-6-oxo-4,4'-bipyrimidin-2-yl)-morpholin-2-yl) -Phenyl)acetamide (compound number 40)
[0553] N-(4-((2S)-morpholin-2-yl)phenyl)acetamide (5.78 g, 26.2 mmol), 2-chloro-1,6-dihydro-1-methyl-6- A solution of oxo-4,4'-bipyrimidine (4.00 g, 18 mmol) and triethylamine (6.00 g, 60 mmol) in THF (100 mL) was stirred at 95°C for 1 hour. The solvent was removed in vacuo and the residue was partitioned between water and dichloromethane. The organic layer was dried over anhydrous sodium sulfate and the solvent was removed under reduced pressure. The resulting residue was purified by silica gel column chromatography (chloroform-methanol, 10:1) to obtain N-(4-((2S)-4-(1,6-dihydro-1- Methyl-6-oxo-4,4'-bipyrimidin-2-yl)-morpholin-2-yl)-phenyl)acetamide (5.7 g, 30%).
Embodiment 3
[0555] (S)-2-(2-(4-(N-cyclohexyl-N-methylamino)phenyl)morpholin-4-yl)-3-methyl-6-(4-pyridyl)-pyrimidine -4-one (Compound No. 10)
[0556] (2S)-2-(4-(cyclohexylamino)phenyl)-4-((1R)-1-phenylethyl)morpholine
[0557] Add (2S)-2-(4-bromophenyl)-4-((1R)-1-phenylethyl)morpholine (7.62 g, 22.0 mmol), palladium acetate (198 mg, 0.88 millimol), 2-(di-tert-butylphosphine)biphenyl (525 mg, 1.76 mmol) and sodium tert-butoxide (2.96 g, 30.8 mmol) in toluene (40 ml) suspension was added cyclohexylamine ( 3.78 mL, 33.0 mmol). After heating at 90°C for 2.5 hours, the resulting suspension was passed through a column of celite. The filtrate was concentrated under reduced pressure, and the residue was purified by silica gel chromatography (eluent: 5%-25% ethyl acetate in hexanes) to give (S)-2-(4-(cyclo Hexylamino)phenyl)-4-((R)-1-phenethyl)morpholine (6.95 g, 87%).
[0558] (2S)-2-(4-(N-cyclohexyl-N-methylamino)phenyl)-4-((1R)-1-phenylethyl)morpholine
[0559] Add (S)-2-(4-(cyclohexylami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com