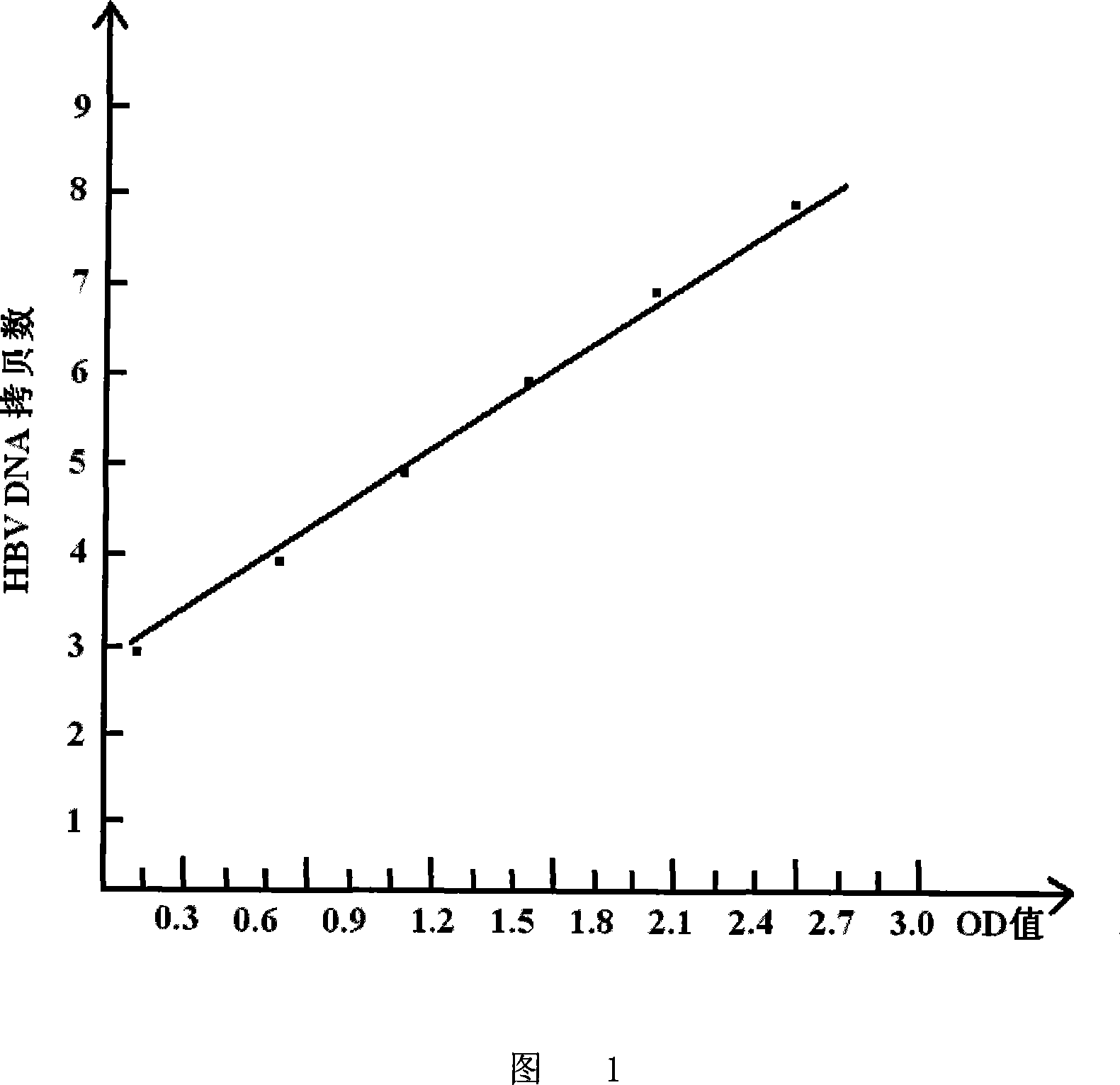HBV-DNA kit for quantitative enzyme-linked measurment of heaptitis B core antigen
A technology of core antigen and ELISA, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of unable to reflect the real state of the virus, the immune response of patients, the existence of hepatitis B virus, and the complicated operation. Achieve the effect of stable specificity, easy operation and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Preparation of Hepatitis B Core Antigen ELISA Quantitative Assay Kit for HBV DNA
[0056] 1. Preparation and purification of anti-HbsAg polyclonal antibody:
[0057] Immune rabbits (purchased from Beijing Experimental Animal Research Center) with HbsAg (purchased from Beijing Medico Biotechnology Co., Ltd.) to obtain antiserum; add saturated ammonium sulfate to the antiserum to a final concentration of 33%, and stir for 1 hour; Centrifuge at 4000g for 15 minutes, remove the supernatant; re-dissolve the precipitate with the same volume of saturated ammonium sulfate as above and remove the precipitate; centrifuge at 4000g for 15 minutes, discard the supernatant, dissolve and dialyze with an appropriate phosphate buffer to obtain the crude polyclonal antibody, and then DEAE column chromatography removes impurity proteins (DEAE fiber chromatography column is purchased from PHAMACIAL company), pre-equilibrates the DEAE column with PB buffer, then elutes, collects ...
Embodiment 2
[0083] Embodiment 2: The usage method of HBcAg quantitative determination HBV DNA
[0084] 1. Detection
[0085] 1. Take out the HBV surface antibody polyantibody pre-coated reaction strip from the kit, place it at room temperature for a period of time, until the temperature of the strip is consistent with room temperature, wash twice with distilled water, and pat dry.
[0086] 2. Add 100 microliters of each concentration of HBV DNA serum standard, test serum, negative serum, and positive serum to the reaction wells, set a blank well for each experiment, add 100 microliters of buffer solution, and react at 45°C for 30 minutes.
[0087] 3. Discard the liquid in the well, wash 7 times with distilled water, and pat dry.
[0088] 4. Add shell-opening agent: add 3 drops of shell-opening agent to each well (not add to blank well) and incubate at 45°C for 30 minutes (note: do not pour this solution away).
[0089] 5. Take out the HBV core antibody (HBcAb) polyclonal antibody pre-co...
Embodiment 3
[0100] Embodiment 3: HBcAg ELISA quantitative determination of HBV DNA application and fluorescent quantitative PCR detection of HBV DNA comparison
[0101] 1. Sources of clinical specimens:
[0102] The inventor collected 152 positive sera from the Huainan Institute of Liver Diseases and Beijing You'an Hospital, including 79 cases of major three yang, 73 cases of small three yang, and 43 completely negative sera; a total of 195 serums. These sera were then measured simultaneously with the method of the present invention and a commercial fluorescent quantitative HBV DNA PCR detection kit.
[0103] 2. Determination method:
[0104] HBcAg ELISA for quantitative determination of serum HBV DNA was operated according to the instruction manual (self-made kit) (the method was the same as in Example 2), and the fluorescent quantitative HBV DNA PCR assay was operated according to the manual of the fluorescent quantitative PCR detection kit produced by Guangzhou Zhongshan Daan Gene Tec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com