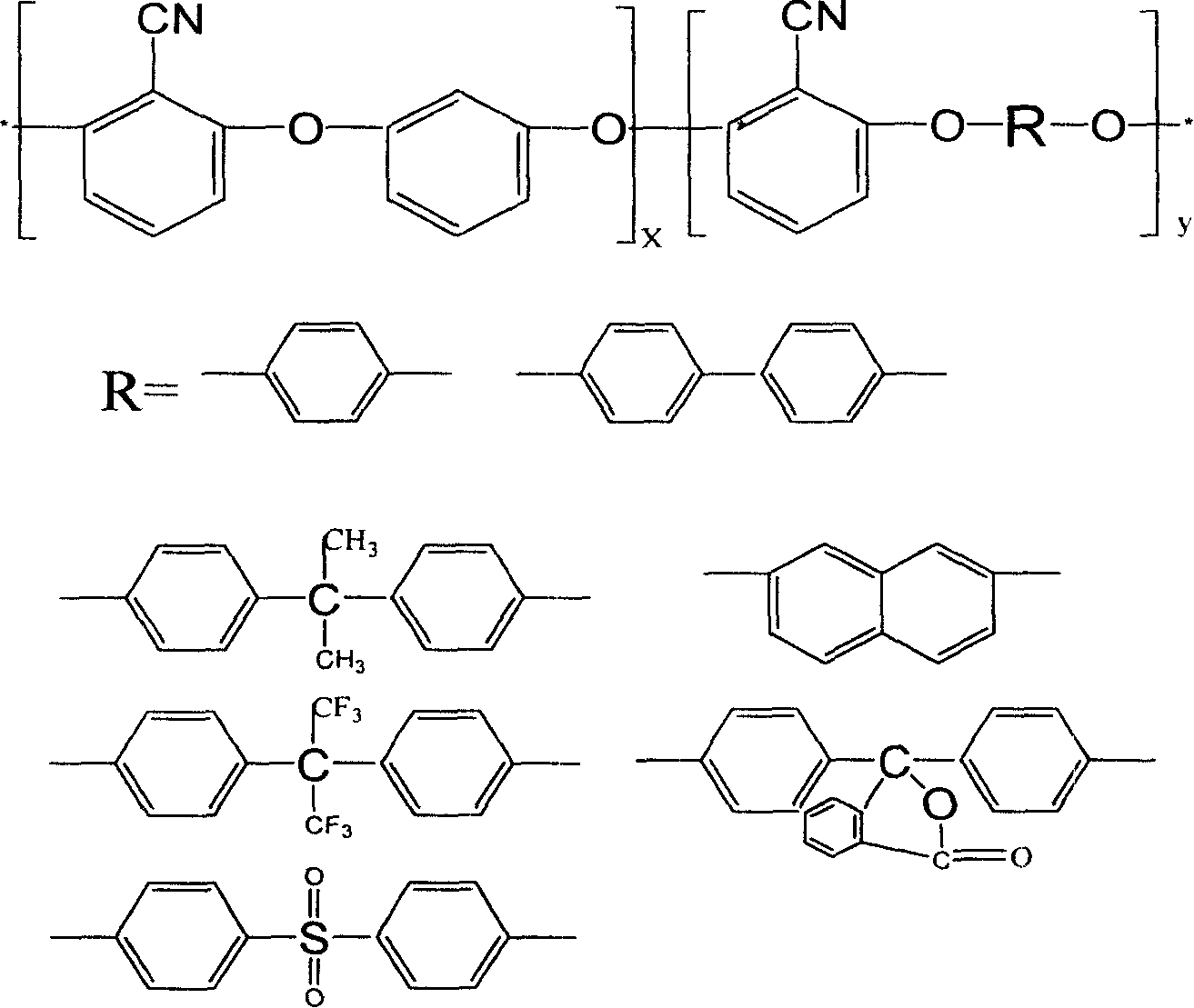
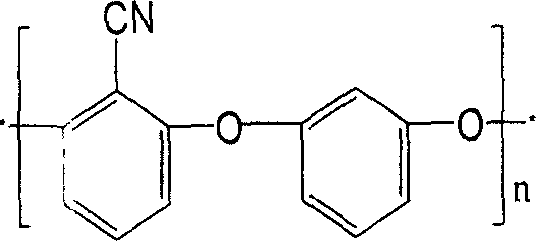
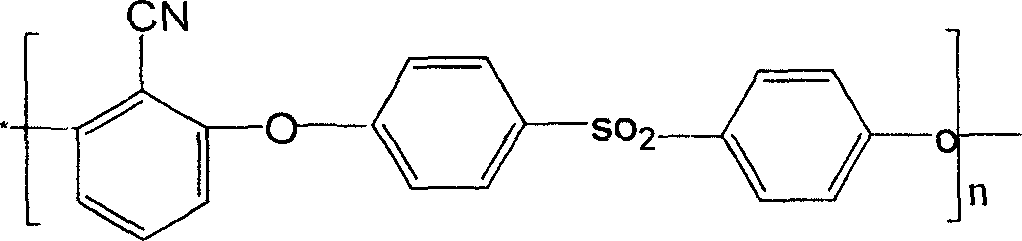
Copolymer of polyarylether nitrile containing chian element of iso-benzene and preparation method
A technology of polyarylene ether nitrile and m-phenylene chain link, which is applied in the field of engineering plastics, can solve the problems of monotonous performance, poor interactive adjustment between material structure and performance, etc., and achieve increased molecular weight, significant economic and social benefits, and high performance-price ratio Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 polyarylether nitrile copolymer of the present invention
[0034] with separator, stirrer, thermometer and N 2 Add 2 moles (344 grams) of dichlorobenzonitrile, 110 grams of resorcinol (1 mole), 1 mole of hydroquinone (110 grams), and 20 moles of N-methylpyrrolidone in the four-necked bottle of import and export. (1980 grams), 2.17 moles (300 grams) of anhydrous potassium carbonate, 4.3 moles (400 grams) of toluene, pass into N 2 , start stirring, heat up to 120-160°C for dehydration reaction for 2-5 hours, distill off the toluene, raise the temperature to 180-200°C, and react for 2-5 hours. Poured into water while hot, precipitated, acidified with dilute hydrochloric acid to pH 7, filtered, the dried crude product was dissolved in dimethylformamide, the filtered clear was precipitated in water / methanol, and finally dried in vacuum to obtain Polyarylether nitrile copolymer of m-phenylene chain, yield 98%, 10-20% solubility in DMF, DMAc, ...
Embodiment 2
[0035] The preparation of embodiment 2 polyarylene ether nitrile copolymers of the present invention
[0036] with separator, stirrer, thermometer and N 2 Add 2 moles of dichlorobenzonitrile (344 grams), 1 mole of resorcinol (110 grams), 1 mole of phenolphthalein (314 grams), and 30.3 moles of N-methylpyrrolidone (3000 grams) in the four-necked bottle of import and export. ), anhydrous potassium carbonate 2.17 moles (300 grams), toluene 4.3 moles (400 grams), pass into N 2 , start stirring, heat up to 120-160°C for dehydration reaction for 2-5 hours, distill off the toluene, raise the temperature to 180-200°C, and react for 2-5 hours. Poured into water while hot, precipitated, acidified with dilute hydrochloric acid to pH 7, filtered, the dried crude product was dissolved in dimethylformamide, the filtered clear was precipitated in water / methanol, and finally dried in vacuum to obtain The polyarylether nitrile copolymer of m-phenylene chains has a yield of 97%, has a solubil...
Embodiment 3
[0037] Embodiment 3 The preparation of polyarylether nitrile copolymer of the present invention
[0038] with separator, stirrer, thermometer and N 2Add 2 moles of dichlorobenzonitrile (344 grams), 1 mole of resorcinol (110 grams), 1 mole of biphenol (186 grams), and 30.3 moles of N-methylpyrrolidone in the four-necked bottle of import and export. (3000 grams), anhydrous potassium carbonate 2.17 moles (300 grams), toluene 4.3 moles (400 grams), pass into N 2 , start stirring, heat up to 120-160°C for dehydration reaction for 2-5 hours, distill off the toluene, raise the temperature to 180-200°C, and react for 2-5 hours. Poured into water while hot, precipitated, acidified with dilute hydrochloric acid to pH 7, filtered, the dried crude product was dissolved in dimethylformamide, the filtered clear was precipitated in water / methanol, and finally dried in vacuum to obtain Polyarylether nitrile copolymer of m-phenylene chain, yield 99%, 10-15% solubility in DMF, DMAc, NMP and o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com