Oral recombined DNA vaccine for accelerating growth of animal, and application
A DNA vaccine and animal growth technology, applied in the field of animal genetic engineering, can solve the problems of unfavorable growth of large animals, affect the immune effect, and the inability of viruses to replicate in large quantities, so as to achieve the effect of increasing daily weight gain and feed utilization, and improving the immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Construction of eukaryotic expression plasmid pGM-CSF
[0039] 1.1 Reverse transcription and PCR amplification
[0040] Using the RT-PCR kit (refer to the instruction manual of the RNA PCR Kit (AMV) Ver.3.0 kit of TaKaRa) to take 1-2 μg of the extracted total cellular RNA for reverse transcription, and react 10 μL total volume containing:
[0041] MgCl 2 2μL
[0042] OligodT primer (2.5pmol / μL) 0.5μL
[0043] dNTP Mixture (each 10mM) 1μL
[0044] RNase inhibitor (40U / μL) 0.25μL
[0045] Reverse transcriptase (5U / μL) 0.5μL
[0046] 10×RT Buffer 1μL
[0047] RNase Free dH 2 O to bring the total reaction volume to 10 µL.
[0048] Perform reverse transcription reaction under the following conditions: 30°C for 10min
[0049] 42℃30min
[0050] 99℃5min
[0051] 5℃5min
[0052] React for 1 cycle. The RT product was stored at -20°C for later use.
[0053] Add 10 μL of the reve...
Embodiment 2
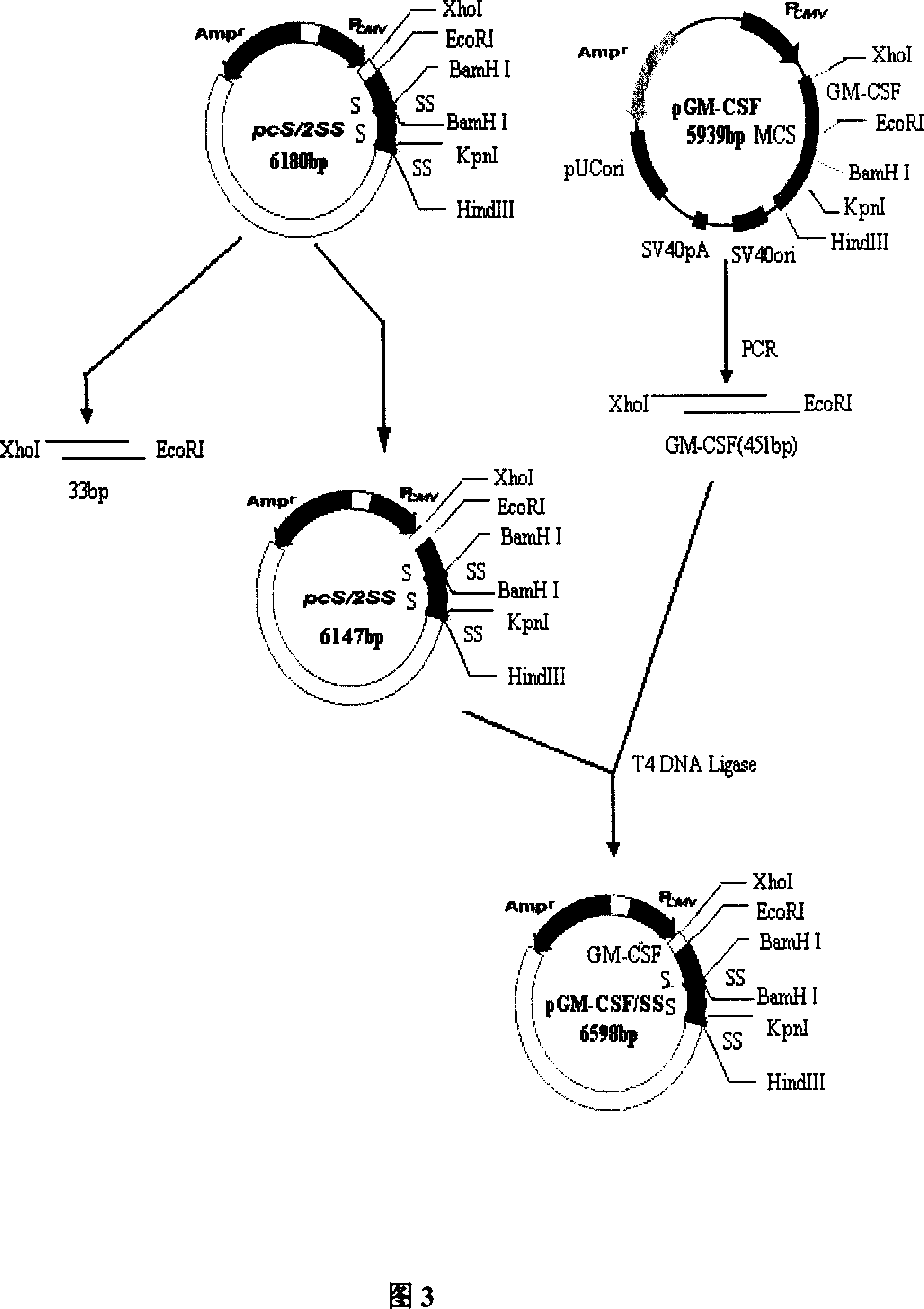
[0082] Example 2 Construction of Fusion Expression Plasmid pGMCSF-SS
[0083] 2.1 Extraction and purification of plasmid pGM-CSF
[0084] Pick a single colony of newly activated pGM-CSF from the LB plate, inoculate it into 10 mL of LB liquid medium, cultivate overnight at 37°C and 240 rpm, dilute it into an appropriate volume of LB at a volume ratio of 1:250, and continue culturing for 12 hours. The bacteria were collected by centrifugation, and the pGM-CSF plasmid was extracted and purified (refer to the instruction manual of the plasmid extraction kit of Shanghai Huashun Biological Co., Ltd.).
[0085] 2.2 Amplification of GM-CSF coding gene
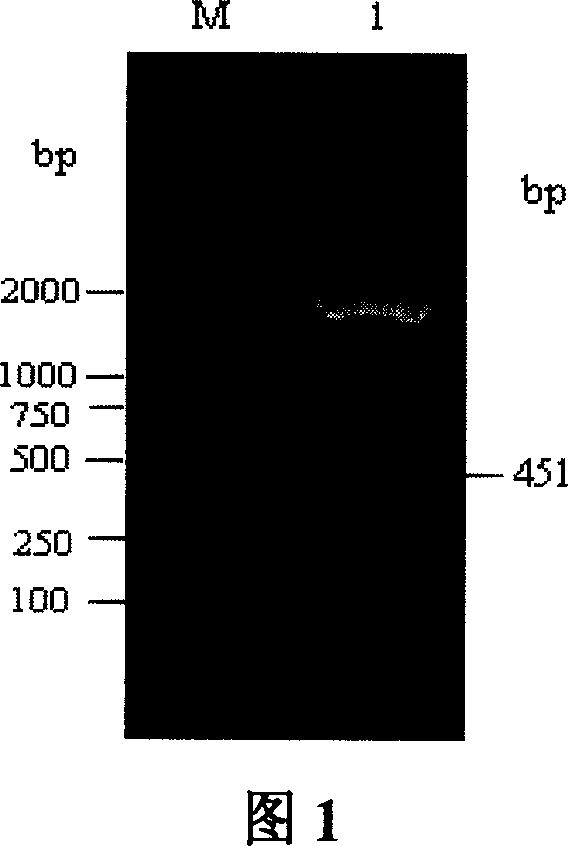
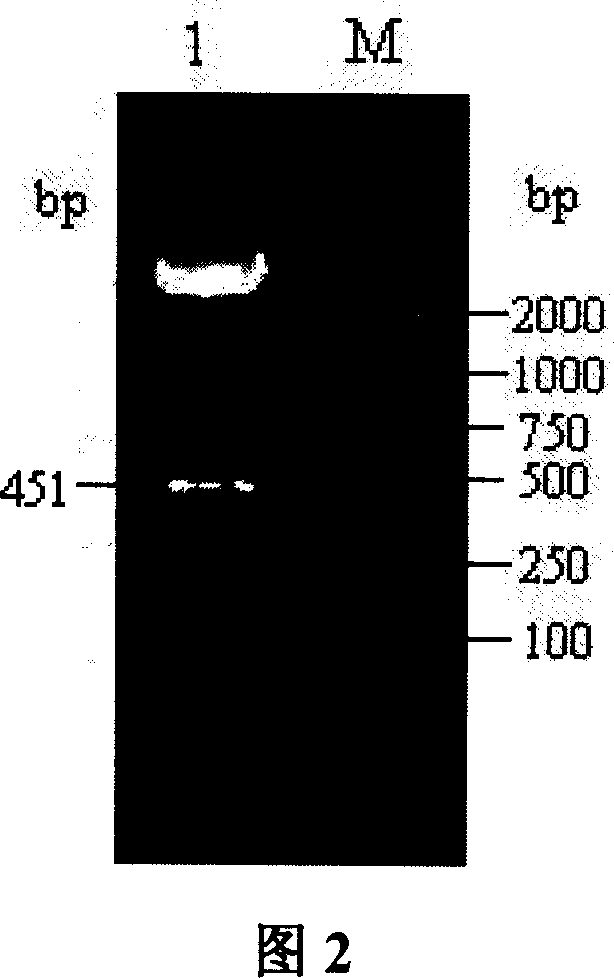
[0086] Referring to accompanying drawing 4, using Primer 5.0 primer design software, the GMCSF gene was obtained by PCR using the porcine eukaryotic expression plasmid pGM-CSF (Example 1) as a template. The upstream primer (P1) is: 5'-GTAT CTCAGAAGGATGTGGC-3', introduced the restriction endonuclease XhoI site (the sequence in the b...
Embodiment 3
[0101] Example 3 Preparation of Oral Recombinant DNA Vaccine Using Attenuated Salmonella Typhimurium as a Vector
[0102] 3.1 pGMCSF-SS plasmid transformation of attenuated Salmonella typhimurium
[0103] The same method as above was used to prepare fresh competent attenuated Salmonella typhimurium, and the fusion expression plasmid pGMCSF-SS was transformed into competent attenuated Salmonella typhimurium (the specific operation steps were the same as in Example 1.3).
[0104] The attenuated Salmonella typhimurium (Salmonella typhimurium) CSO22 / pGMCSF-SS containing the fusion expression plasmid was deposited in China Center for Type Culture Collection (CCTCC) on December 19, 2006, and the preservation number is CCTCC M206141.
[0105] 3.2 Identification of plasmids in recombinant Salmonella typhimurium
[0106] Pick the single colonies of positive clones obtained in step 3.1 and inoculate them in Amp-containing LB liquid medium and culture them overnight at 37°C, and extract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com