Drug for monitoring worsening of hepatitis
A technology for hepatitis and severe disease, which is applied in the monitoring field of severe hepatitis and can solve unknown problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
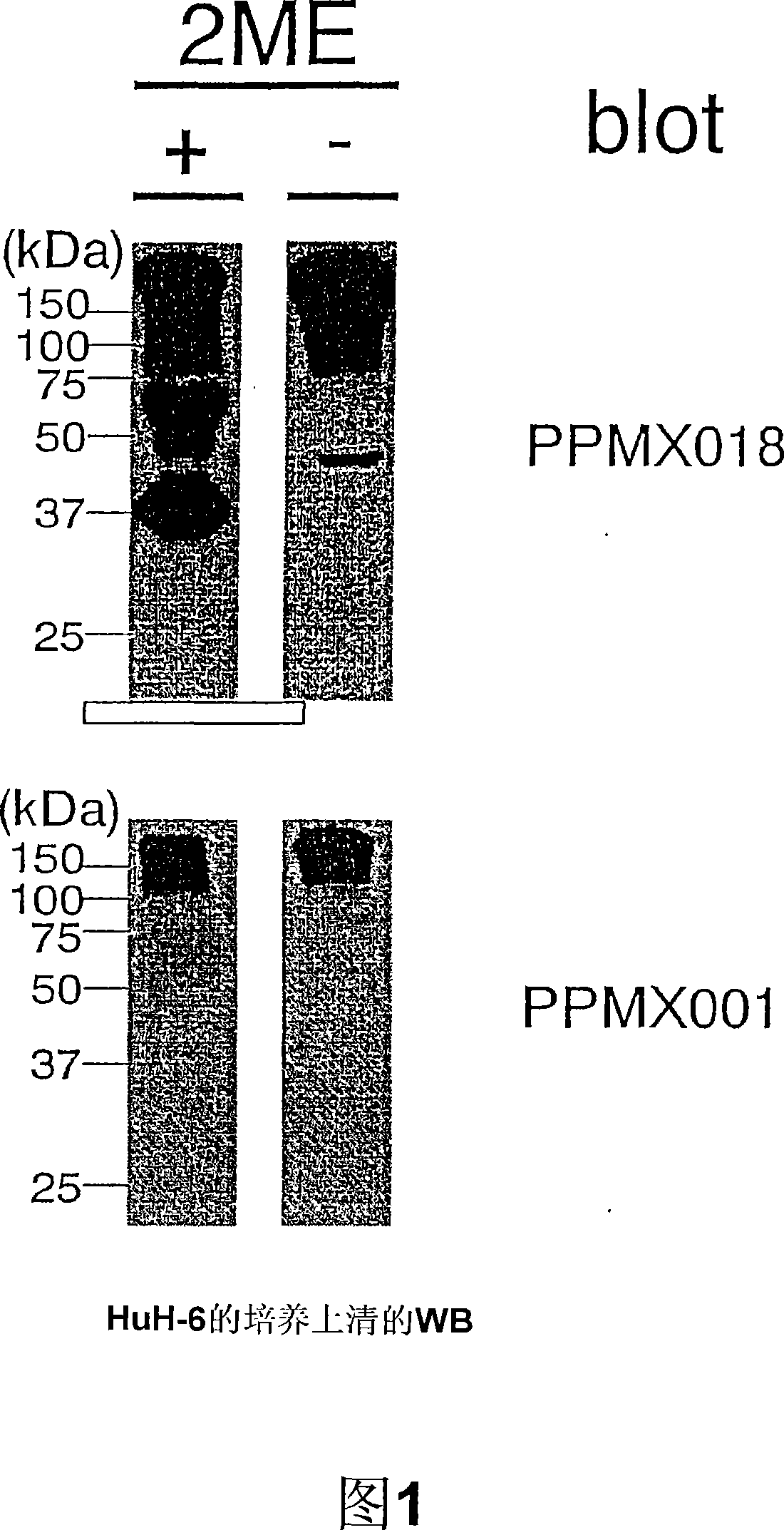
[0106] The acquisition of embodiment 1 natural type GPC3
[0107] HuH6 cells were cultured in large quantities using a dish with a diameter of 150 mm, and the culture supernatant was collected and purified. The culture supernatant was filled with DEAE Sepharose Fast Flow (Amersham CAT#17-0709-01), washed, and eluted with a buffer containing 500 mM NaCl. Next, after concentration with Centriprep-10 (Millipore CAT#4304), purification was carried out by gel filtration with Superdex 200HR 10 / 30 (Amersham CAT#17-1088-01) to obtain crude and purified GPC3.
[0108] In order to obtain high-purity GPC3, use a monoclonal antibody made of crude and purified GPC3 as an antigen to prepare an affinity column, fill it with HuH-6 crude and purified GPC3, and then wash it with pH 3.5, 0.1M glycine hydrochloric acid After that, in 4M MgCl 2 ·6H 2 O, and then dialyzed against 50 mM Tris-HCl (pH 7.5), 150 mM NaCl.
Embodiment 2
[0109] The acquisition of embodiment 2 recombinant GPC3
[0110]Use the plasmid DNA containing the full-length human GPC3 cDNA to construct the flag-added soluble GPC3, that is, construct the plasmid DNA expressing the recombinant GPC3 cDNA. A downstream primer (5'-ATAGAA TTC CAC CAT GGC CGG GAC CGT GCG C-3' (SEQ ID NO: 1)) designed to remove the hydrophobic region (564-580 amino acids) on the C-terminal side and an EcoRI recognition sequence, The upstream primer of the Kozak sequence (5'-ATA GGA TCC CTTCAG CGG GGA ATG AAC GTT C-3' (SEQ ID NO: 2)) was used for PCR. The resulting PCR fragment (1711 bp) was cloned in pCXND2-Flag. The prepared expression plasmid DNA was introduced into CHO cell DXB11 strain, and the CHO strain with high expression of soluble GPC3 was obtained by selection with 500 μg / mL Geneticin.
[0111] Use 1700cm 2 The spinner bottle of the flag-added soluble GPC3 high-expression CHO strain was carried out in large quantities, and the culture supernatant w...
Embodiment 3
[0112] Embodiment 3 Production of anti-GPC3 monoclonal antibody
[0113] Five Balb / C mice (CRL) were immunized with recombinant GPC3 or native GPC3. In the case of recombinant GPC3, the immunization protein was prepared to be 100 μg / mouse at the time of primary immunization; in the case of native GPC3, the immunization protein was adjusted to be 10 μg / mouse. The emulsified drug was subcutaneously administered using FCA (Freund's complete adjuvant (H37Ra), Difco (3113-60), Becton Dickison (cat#231131)). As a booster immunization, after 2 weeks, recombinant GPC3 was adjusted to 50 μg / mouse, and natural GPC3 was adjusted to 5 μg / mouse. The drug prepared above was emulsified with FIA (Freund's incomplete adjuvant, Difco (0639-60), Becton Dickison (cat#263910)), and the emulsified drug was subcutaneously administered. Thereafter, a total of 5 booster immunizations were performed at intervals of one week. For the final immunization, the same amount as the booster immunization was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com