Method of synthesizing zirconium silicate powder at low temperature by unhydrolyzed sol-gel method
A technology of zirconium silicate and gel method, applied in the field of low temperature synthesis of zirconium silicate powder, can solve the problems of high price, difficult industrial production, difficult to obtain, etc., and achieves the effects of low cost, reduced synthesis temperature and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
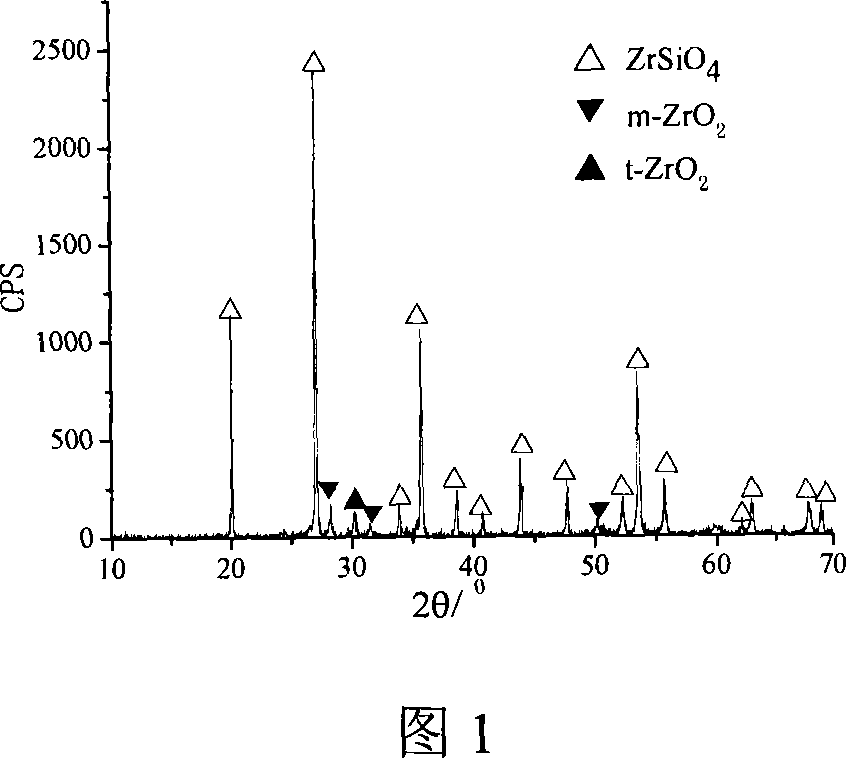
[0019] Si(OC 2 h 5 ) 4 (chemically pure), anhydrous ZrCl 4 (industrial pure) is the precursor, LiF (chemically pure) is the mineralizer, and dichloromethane is the solvent (CH 2 Cl 2 , chemically pure), 0.036molSi(OC 2 h 5 ) 4 , 0.0108molLiF uniformly mixed at room temperature, 0.03mol anhydrous ZrCl 4 with 30mlCH 2 Cl 2 Mix evenly at room temperature, then mix the two mixtures with each other, stir on a magnetic stirrer at room temperature for 10 minutes, continue heating and stirring at 65°C for 20 minutes, and then place them in an oil bath at 110°C for 12 hours under reflux to obtain a transparent precursor sol. Dry the sol in an oven at 35°C to obtain dry glue, and finally crush and calcinate the dry glue. The calcination system is: before the solvent is evaporated at 350°C, the heating rate is 4°C / min, and then it is raised to 700°C at 8°C / min , heat preservation 60min, the synthesized zirconium silicate powder is shown in accompanying drawing 1.
Embodiment 2
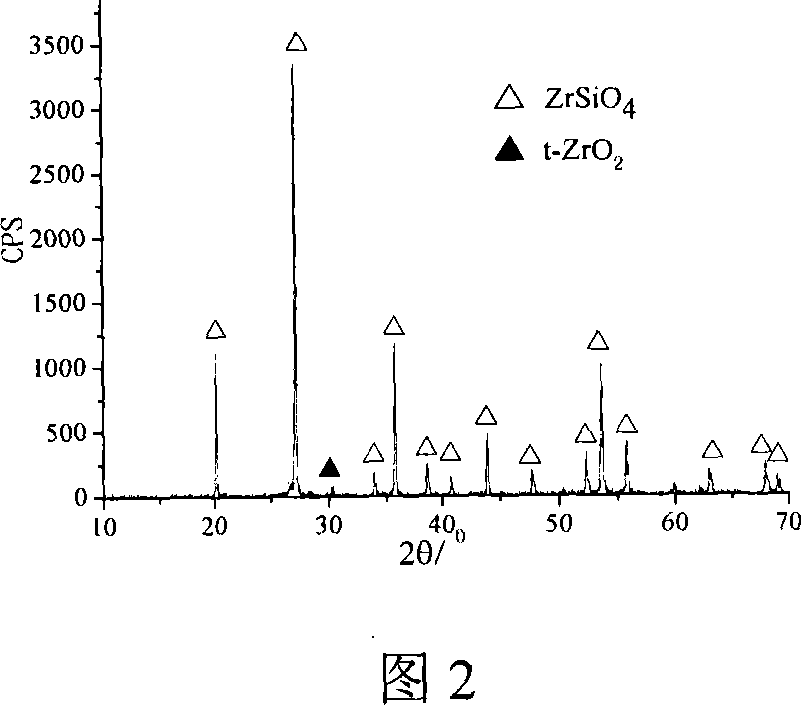
[0021] Si(OC 2 h 5 ) 4 (chemically pure), anhydrous ZrCl 4 (industrial pure) is a precursor, and LiF (chemically pure) is a mineralizer. The consumption of each raw material is the same as that of the preparation process and Example 1, but the solvent used is ethanol (chemically pure), and the drying temperature is 80 ° C. Synthetic See accompanying drawing 2 for zirconium silicate powder.
Embodiment 3
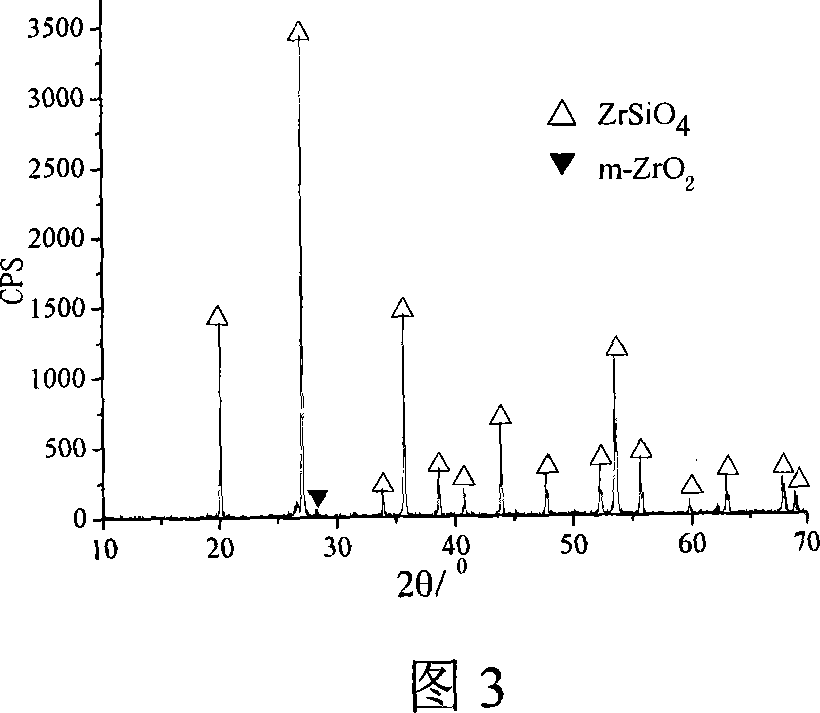
[0023] Si(OC 2 h 5 ) 4 (chemically pure), anhydrous ZrCl 4 (industrial pure) is the precursor, LiF (chemically pure) is the mineralizer, and 0.036molSi(OC 2 h 5 ) 4 Mix evenly with 0.0108mol LiF, then add 0.03mol anhydrous ZrCl 4 Add Si(OC 2 h 5 ) 4 and LiF in a mixed solution, placed on a magnetic stirrer at room temperature and stirred for 10 minutes to obtain a sol, and the sol was heated and stirred at 65°C for 20 minutes, and then placed in an oil bath at 110°C for reflux heating for 12 hours to obtain a dry glue, and finally The dry rubber was crushed and calcined, and the calcining system was as follows: the heating rate was 4°C / min before 350°C, and then raised to 700°C at 8°C / min, and kept for 30 minutes. The synthesized zirconium silicate powder is shown in Figure 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com