Methods of concentrating antibodies and therapeutic products thereof
A technology of antibody and antibody concentration, applied in the direction of antibody medical components, peptide preparation methods, chemical instruments and methods, etc., can solve the problems of low flux, low energy efficiency, low mass transfer rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] In the above-mentioned embodiment of the method for preparing highly concentrated antibodies of the present invention, the following further exemplifies how to prepare and use the preparation method and product of the present invention.
[0040] In an embodiment of the present invention, a method for preparing a highly concentrated antibody composition is provided, for example, the following steps are completed in the stated order, including:
[0041] The first ultrafiltration is performed on the first antibody product, and the concentration of the first antibody product is, for example, about 0.1 to about 10 grams per liter (g / L) to provide the second antibody product as a retentate, which has Higher antibody concentration, for example about 10 to about 50 grams per liter;
[0042] Diafiltration is performed on the obtained second antibody product to provide a diafiltered intermediate antibody product as a retentate, which has about the same concentration as the obtained seco...
Embodiment 1
[0113] High concentration formulation of rhuMAb E25
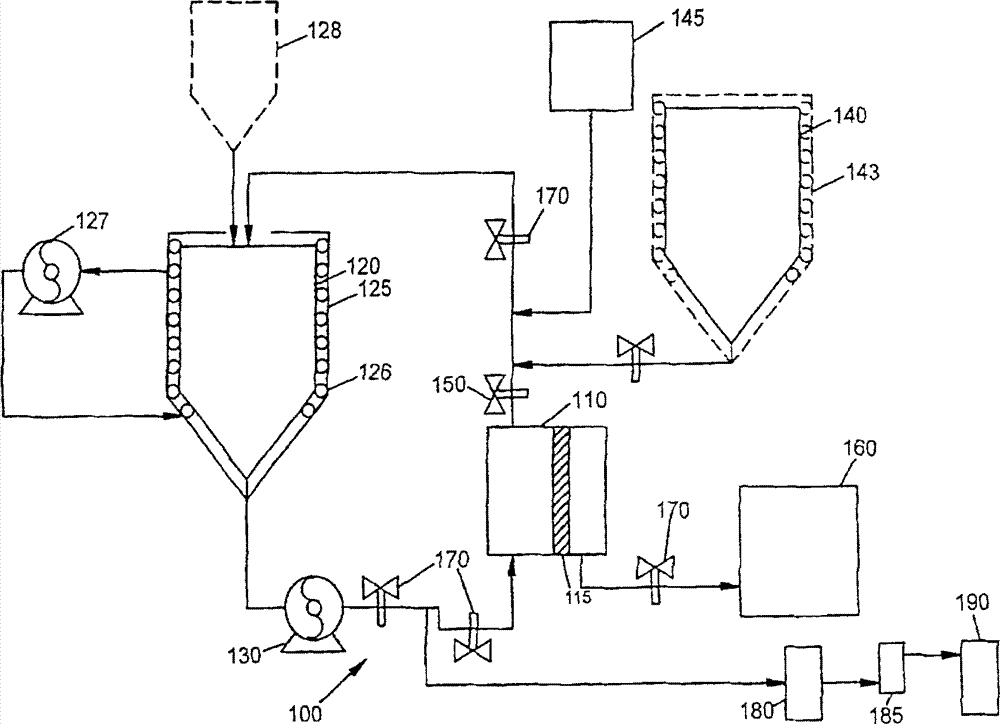
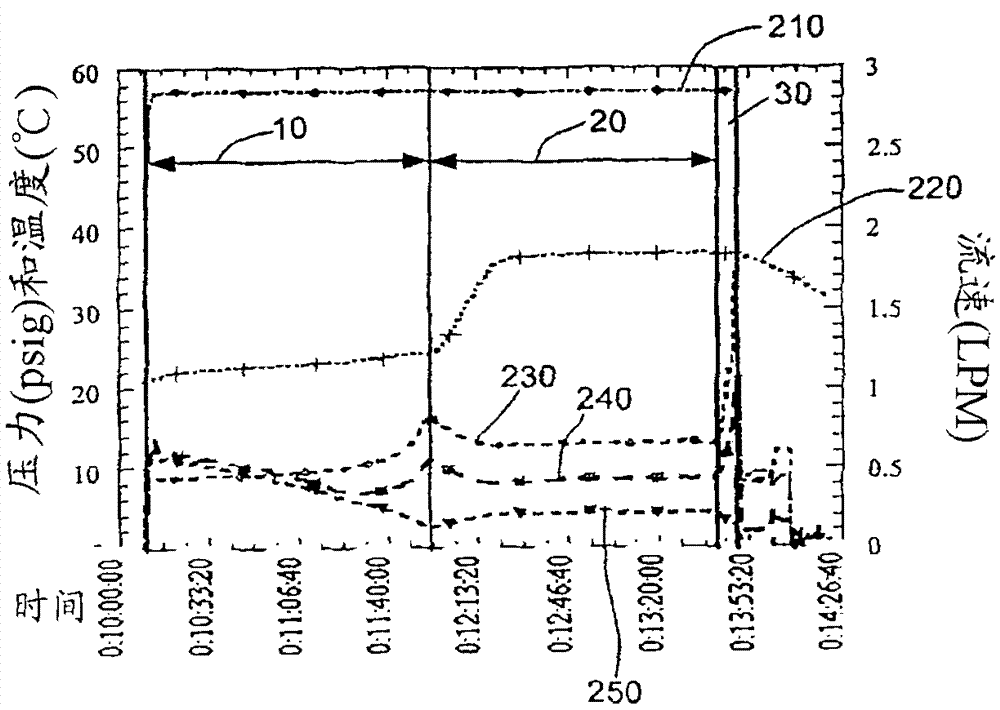
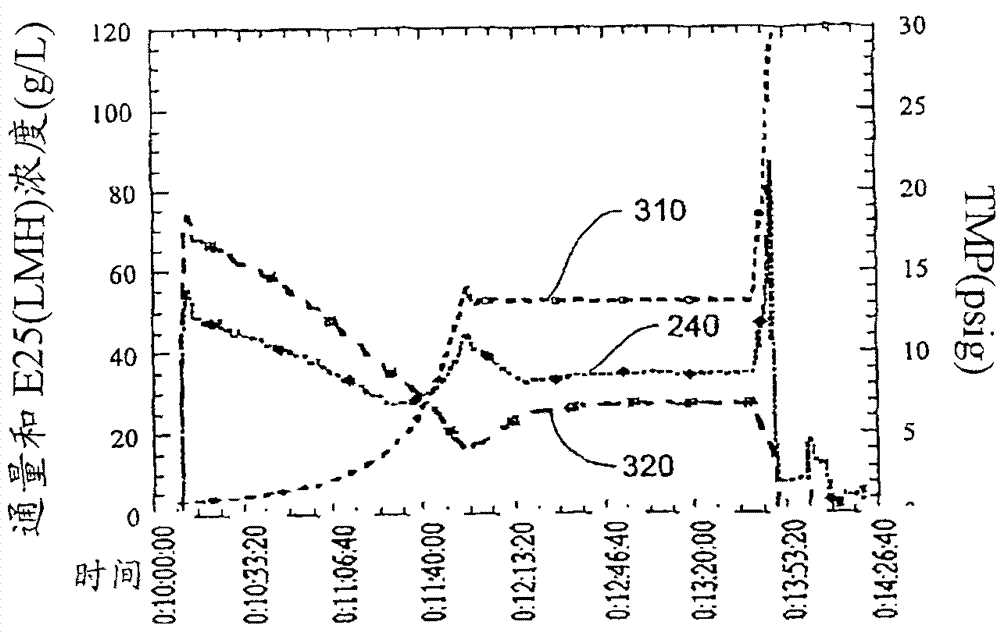
[0114] A pilot scale UF system was used to concentrate / formulate rhuMAb E25 (a recombinant human monoclonal antibody against IgE, US Patent 6,172,213). Assemble a Millipore Pelicon ultrafiltration / diafiltration system with a 5.7 square foot (sqft), 10,000 Dalton regenerated cellulose composite membrane. The system consists of membrane holder, Waukeskaw 6-type rotary vane feed pump, 1 / 2" 316L stainless steel return pipe, and return vessel. Pressure indicator / transmitter (Anderson) is located at the entrance of the membrane holder (FEED), outlet (RETENTATE) and permeate port (FILTRATE). Flow meter (Yokogawa ADMAG) is located at the inlet (FEED) and permeate port (FILTRATE) of the membrane holder. Back pressure regulating valve (Mikroseal) is set at At the outlet of the membrane holder to control the trapped liquid pressure and generate transmembrane pressure (TMP). The reflux container uses a 40-liter 316L tank with a stainless...
Embodiment 3
[0147] Example 3 Preparation of rhuMAb E26 with high concentration using initial feed-batch mode
[0148] Repeat Example 1 with the following changes. The concentrate / formulation is rhuMAb E26 (a recombinant human monoclonal antibody against IgE). The product obtained from this example was used for toxicological analysis. Assemble a Millipore Pelicon ultrafiltration / diafiltration system with 11.4 square feet, 30,000 Dalton regenerated cellulose composite membranes. The flow rate is set to 5.0L / min (0.44L / min / ft 2 ) Constant flow rate. The retentate pressure is maintained between about 6-8 psig during ultrafiltration and diafiltration operations. The total protein material obtained from the previous Q-Sepharose chromatography step was determined to be 6.7 g E26 / L and the volume was 59.3L.
[0149] Since the total material of the feed is larger than the reflux vessel, the UF1 process is started in the feed batch mode. In this mode, the Q-total material is added to the reflux ves...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com