Integration of direct binding sensors with mass spectrometry for functional and structural characterization of molecules
A sensor and molecular mass technology, applied in the direction of instruments, scientific instruments, analytical materials, etc., to achieve the effect of simplifying the mass spectrometry method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Tandem BIND / MALDI-MS test
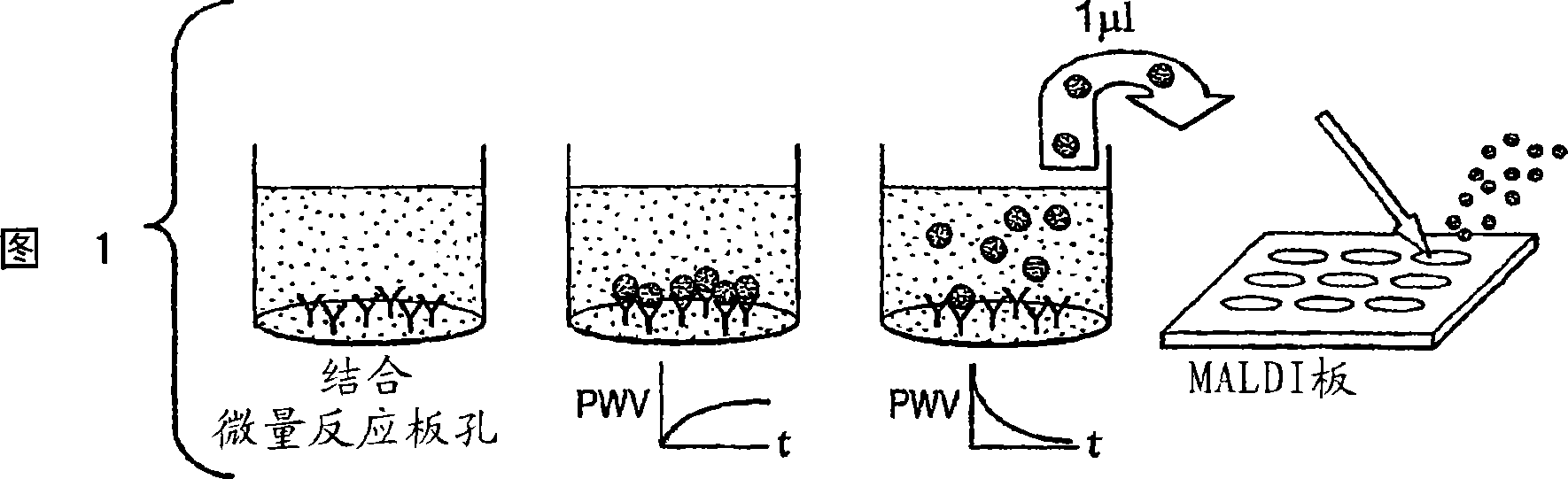
[0072] An assay was performed to show that materials bound to the colorimetric resonant reflectance optical sensor surface can be efficiently eluted and analyzed by mass spectrometry. Allow the capture antibody to be absorbed onto the colorimetric resonant reflectance optical sensor surface. The corresponding antigen is allowed to bind to the antibody during application of the sample on the colorimetric resonant reflectance optical sensor. Any material that is specifically bound to the antibody is then eluted from the surface and a small aliquot of the eluted material is subjected to mass spectrometric analysis. The eluted material was mixed with an appropriate amount of MALDI matrix and added to a MALDI plate for (TOF)MS analysis.
[0073] Figure 1 shows the protocol used to combine colorimetric resonant reflectance optical sensor technology with MALDI-type mass spectrometry. The procedure involved adsorption of antibody (0.1 mg / ml) on a ...
Embodiment 2
[0075] Matrix Assisted Laser Desorption Ionization (MALDI) Mass Spectrometry (MS)
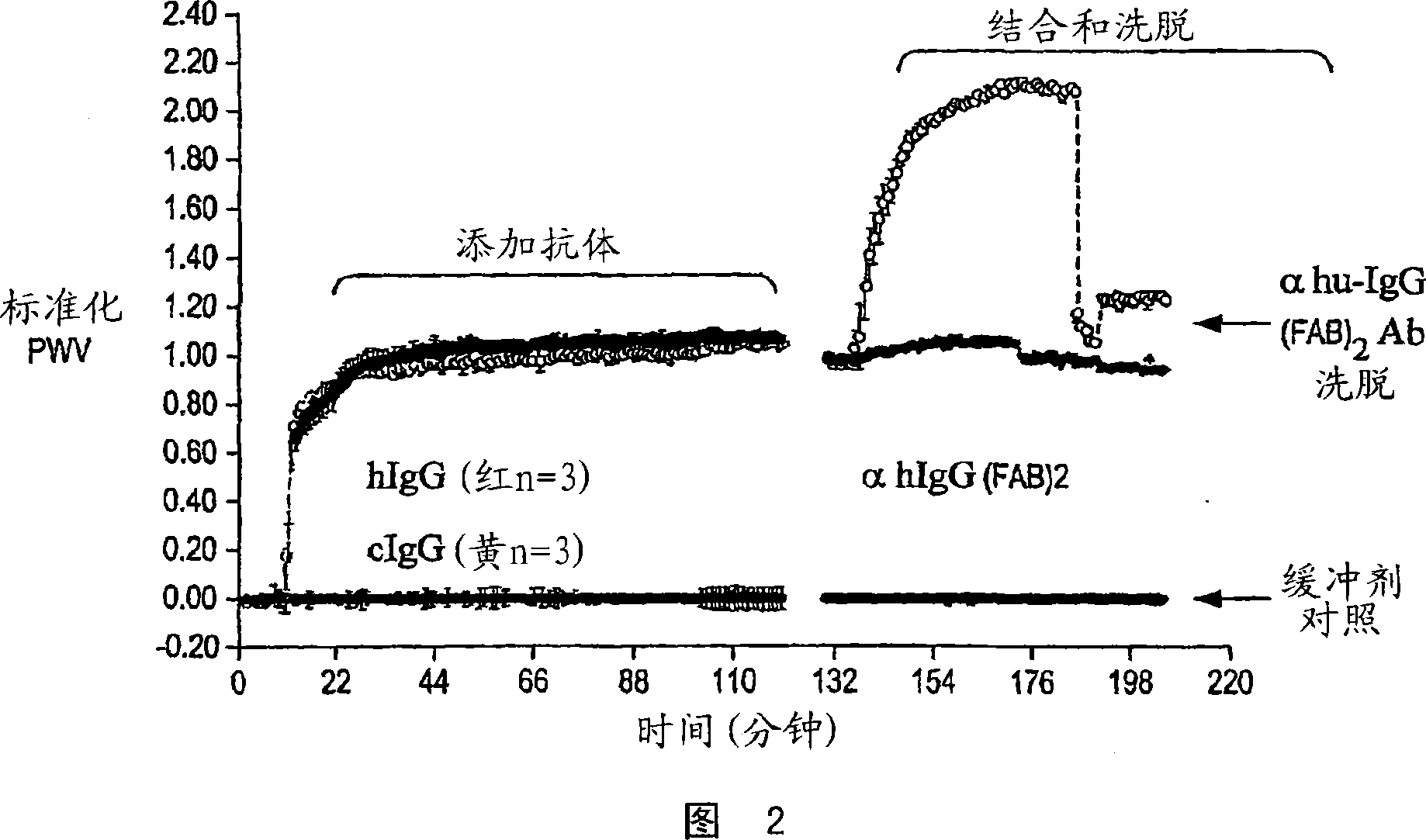
[0076] The colorimetric resonant reflectance optical sensor TiO sensor was pre-rinsed three times with PBS and placed at room temperature for 30 minutes, a baseline reading was taken for a few minutes and 10ul of 1mg / ml human IgG or chicken IgY was diluted into the wells already in the well. 90ul PBS to form a final antibody concentration of 100ug / mL. Proteins were placed into the wells and allowed to bind to the TiO surface for 90 minutes. Unbound protein solution was removed from the wells and the wells were rinsed three times with 200ul of PBS each.
[0077] Another baseline reading is taken for a few minutes, then 1 ul of 1 mg / ml anti-human IgG (Fab) is placed in wells that are coated with hIgG (red) or cIgY (yellow) and allowed to incubate for 60 minutes . All unbound protein solution was removed from the wells, which were rinsed three times with PBS. Monitor the stability of the bound...
Embodiment 3
[0080] Tandem BIND / MALDI-MS Test - MS Data
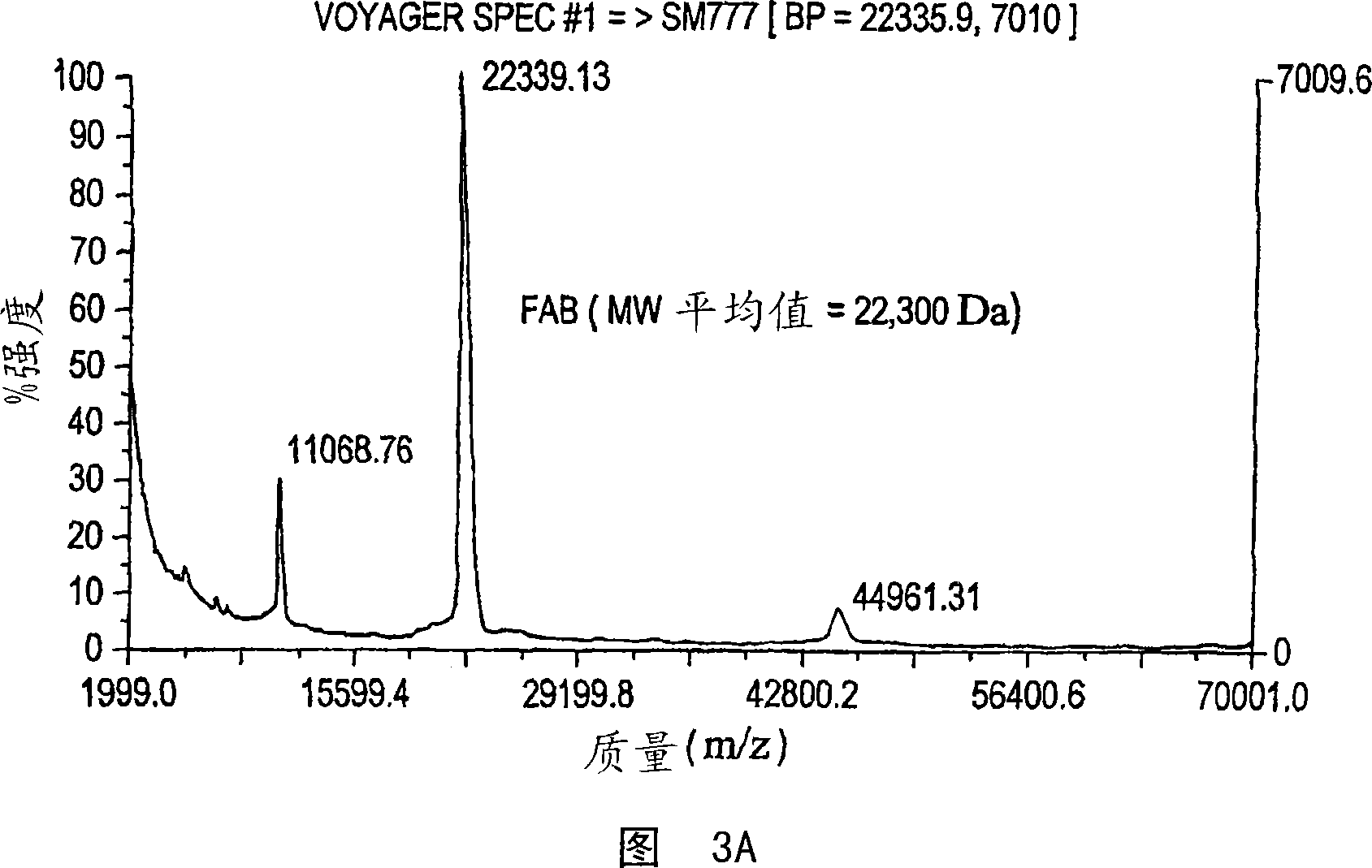
[0081] Figure 3A shows data from a Fab-containing control solution applied to MS prior to exposure under the sensor surface. The main peak is at 22300, and the other two peaks are characteristic peaks related to the parent molecular mass.
[0082] Figure 3B shows MALDI-MS data from the solution eluted from the sensor surface. This mass spectrum is identical to the control spectrum shown in Figure 3A.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com