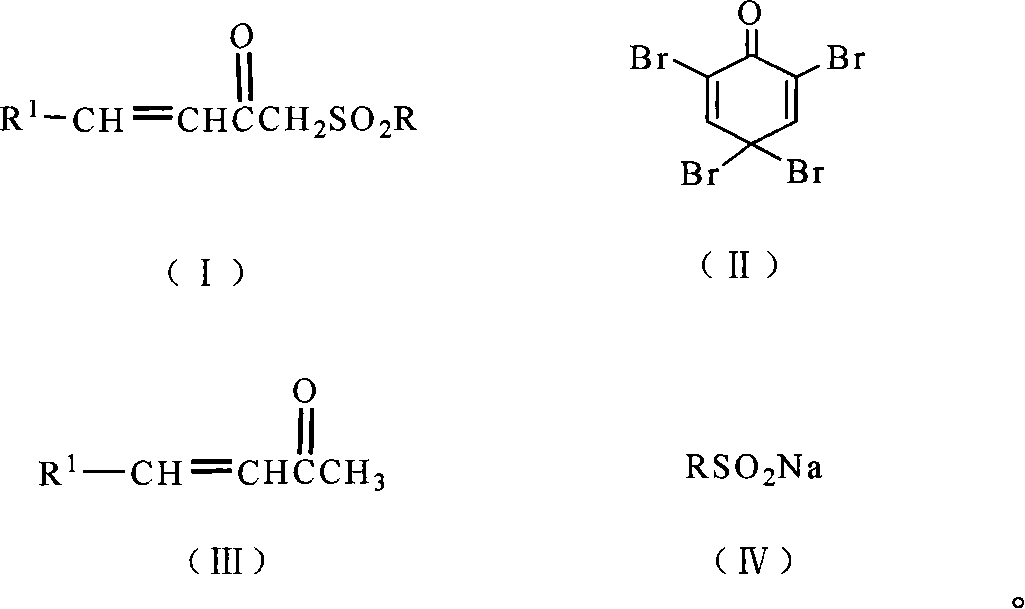
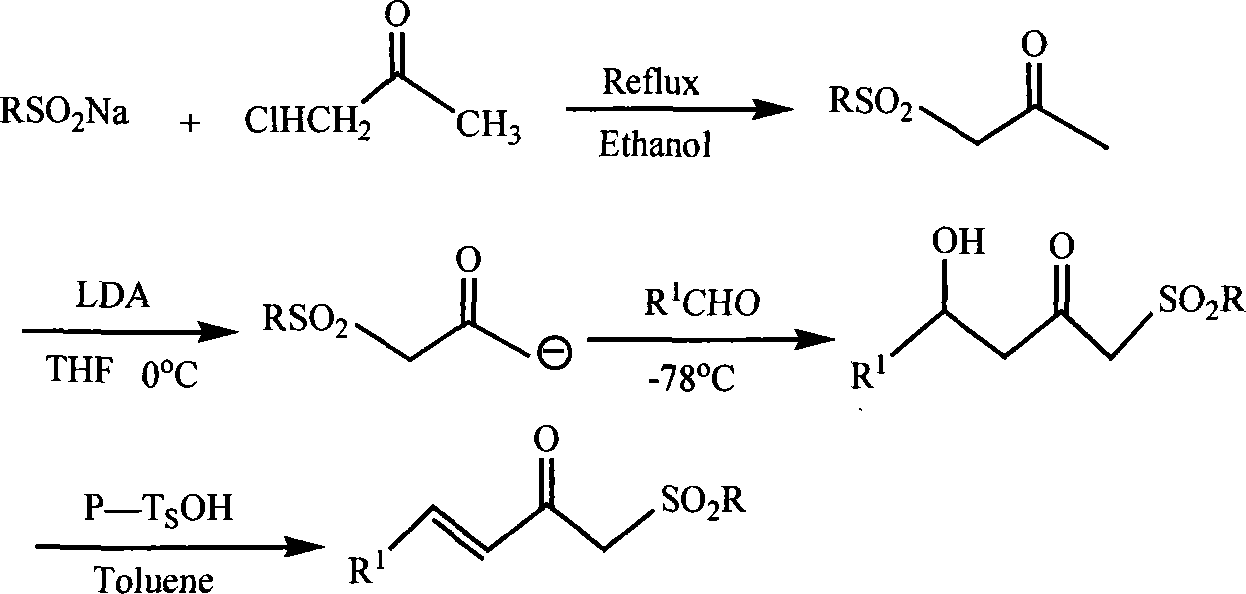
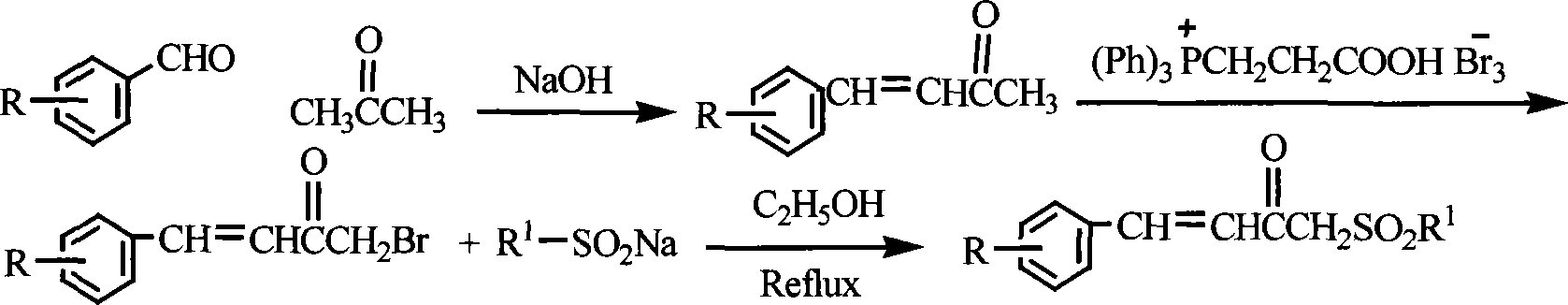
Chemical synthesis method for substituting alpha, beta unsaturated ketone by sulphonyl
A chemical synthesis, unsaturated technology, applied in chemical instruments and methods, organic chemistry, preparation of organic compounds, etc., to achieve the effect of cheap raw materials, cost reduction, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of 4-phenyl-1-benzenesulfonyl-3-butene-2-one
[0029]
[0030] In a 500ml three-necked flask equipped with a mechanical stirring device, add 1.0 g (6.8 mmol) of 4-phenyl-3-buten-2-one, 2,4,4,6-tetrabromo-2,5-cyclohexyl 2.8 g (6.8 mmol) of dien-1-one was then added to 100 ml of 1,4-dioxane dissolved in 1% (g / ml) HCl, and stirred at room temperature for 2 hours. Stop the reaction and saturate with NaHCO 3 Neutralize, CH 2 Cl 2 It was extracted and dried, concentrated, dissolved in 100ml of ethanol, reacted with sodium benzenesulfinate dihydrate, post-treated, and recrystallized from ethanol to obtain 1.15g of the product with a yield of 65%, m.p.96-98°C. 1 H-NMR (CDCl 3 ): δ7.86-7.90 (m, 2H), 7.42-7.67 (m, 9H), 6.94 (1H, d, J=15.8Hz), 4.40 (s, 2H); MS (70ev) m / z: 286 (M + , 7).
Embodiment 2
[0031] Example 2: 4-p-methoxyphenyl-1-benzenesulfonyl-3-buten-2-one
[0032]
[0033] In a 500ml three-necked flask equipped with a mechanical stirring device, add 1.2 g (6.8 mmol) of 4-p-methoxyphenyl-3-buten-2-one, 2,4,4,6-tetrabromo-2, 5.6 g (13.6 mmol) of 5-cyclohexadien-1-one was added, and then 100 ml of acetonitrile dissolved in 5% (g / ml) HCl was added, and stirred at 60° C. for 5 hours. Stop the reaction and saturate with NaHCO 3 Neutralize, CH 2 Cl 2 It was extracted and dried, concentrated, dissolved in 100ml of ethanol, reacted with sodium benzenesulfinate dihydrate, post-treated, and recrystallized from ethanol to obtain 1.7g of the product with a yield of 78%, m.p.142-144°C. 1 H-NMR (CDCl 3 ): δ7.90-7.92(m, 2H), 7.52-7.67(m, 6H), 6.92-6.94(d, J=8.8Hz, 2H), 6.79-6.83(d, J=15.7Hz, 1H), 4.37(s, 2H), 3.86(s, 3H); MS(70ev) m / z(rel.intensity,%) 316(M + ,twenty four).
Embodiment 3
[0034] Example 3: 4-(4'-N, N-dimethylamino)phenyl-1-benzenesulfonyl-3-butene-2-one
[0035]
[0036]In a 500ml three-necked flask equipped with a mechanical stirring device, add 1.3g (6.8mmol) of 4-p-dimethylaminophenyl-3-buten-2-one, 2,4,4,6-tetrabromo-2 , 2.8g (6.8mmol) of 5-cyclohexadien-1-one, and then add 100ml of 1,4-dioxane dissolved in 0.1% (g / ml) HBr, and stir at 100°C for 1 hour. Stop the reaction and saturate with NaHCO 3 Neutralize, CH 2 Cl 2 It was extracted and dried, concentrated, dissolved in 100ml of ethanol, reacted with sodium benzenesulfinate dihydrate, post-treated, and recrystallized from ethanol to obtain 1.0 g of the product with a yield of 54%, m.p.157-159°C. 1 H-NMR (CDCl 3 ): δ7.92-7.90 (m, 2H), 7.44-7.65 (m, 6H), 6.69-6.67 (m, 3H), 4.35 (s, 2H), 3.06 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com