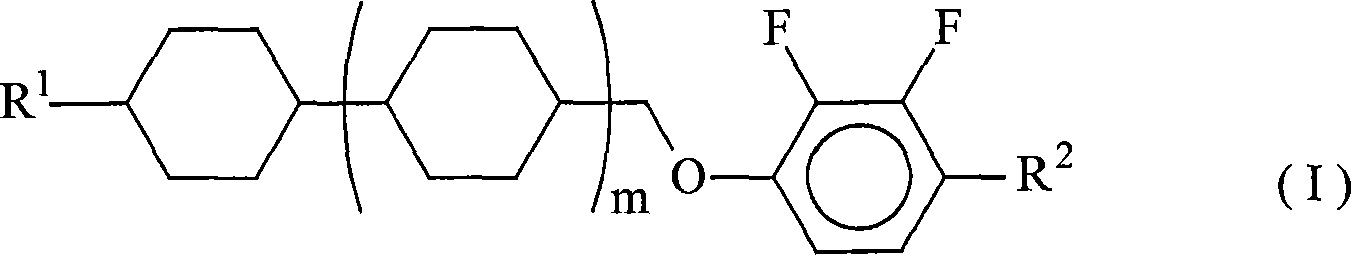
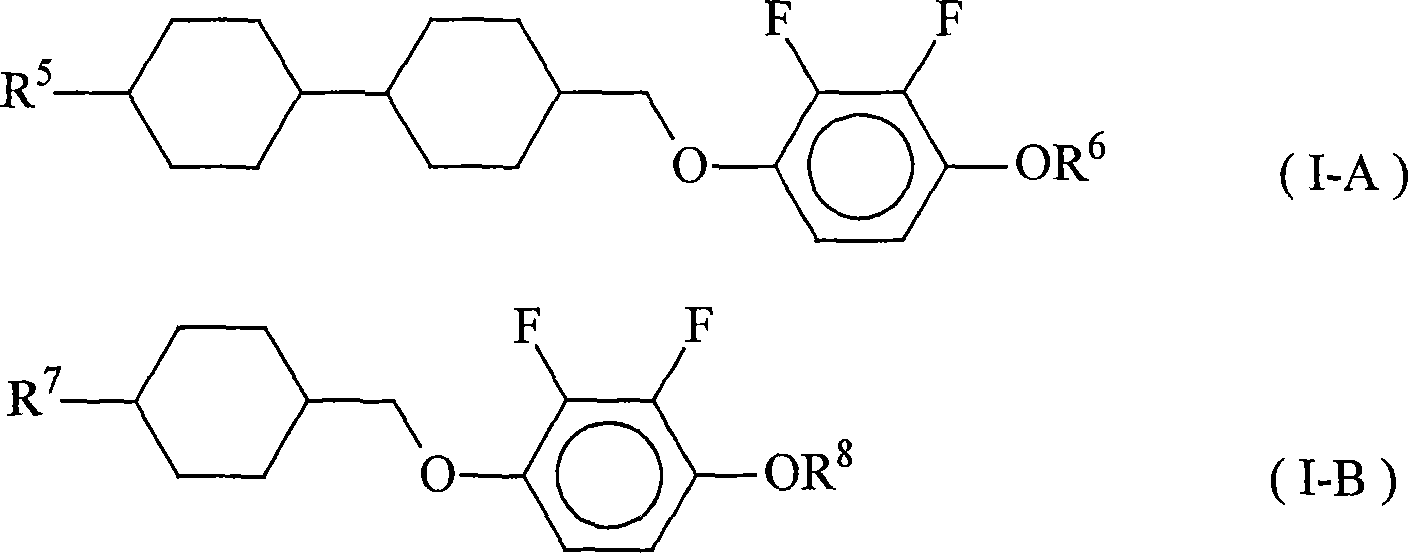
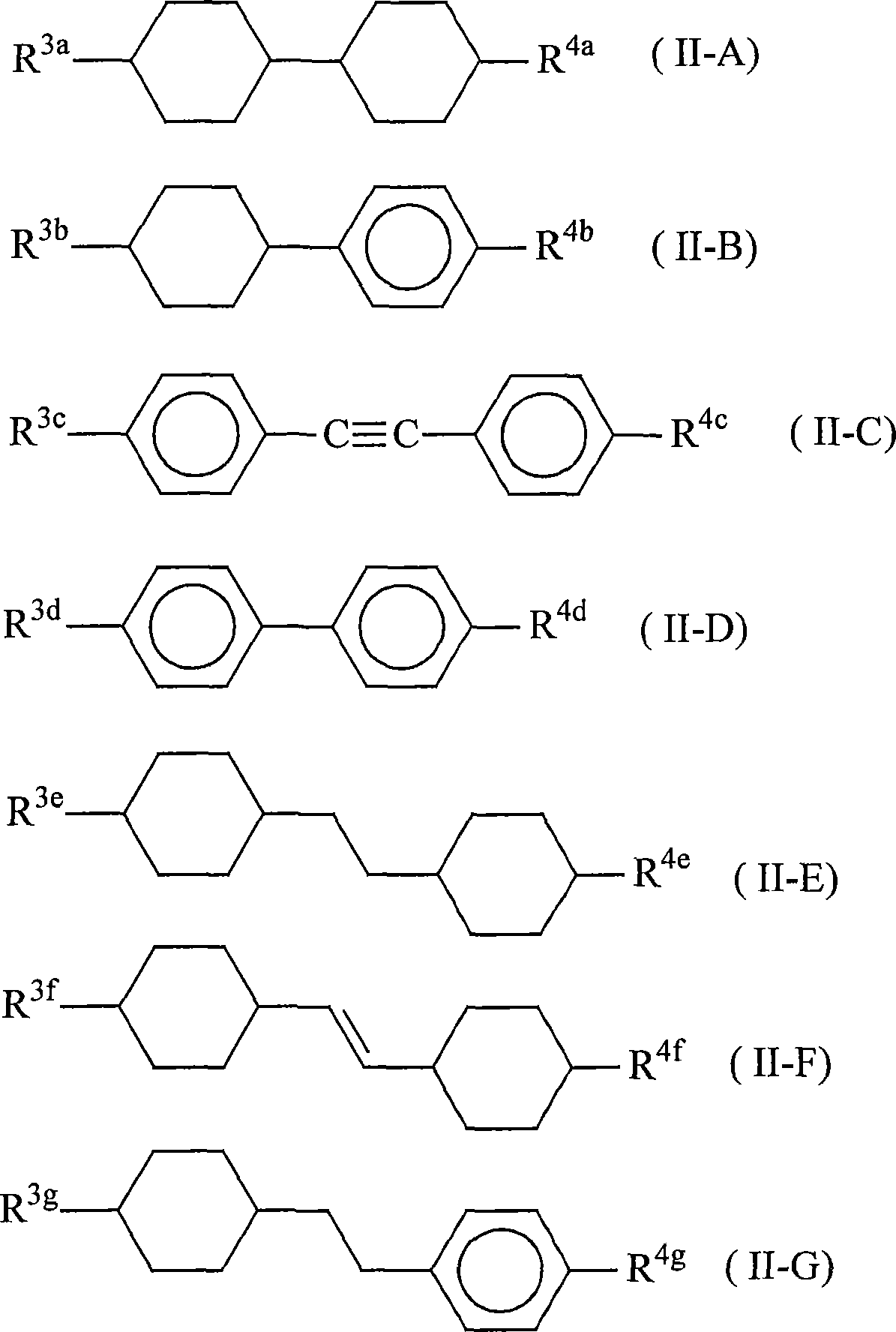
Nematic liquid crystal composition and liquid crystal display element using the same
A technology of liquid crystal composition and compound, which is applied in the direction of liquid crystal materials, instruments, chemical instruments and methods, etc., which can solve the problems that the voltage retention rate cannot be used for active matrix applications, slowness, etc., and achieve the effect of realizing high-speed response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202] (Embodiment 1) 1-ethoxy group-2, the synthesis of 3-difluoro-4-(trans-4-vinylcyclohexyl)methoxybenzene (Ia)
[0203] (1-1) Synthesis of 2,3-difluoro-4-ethoxyphenol
[0204] [chem 34]
[0205]
[0206] (1-1-1) Synthesis of 2,3-difluoroethoxybenzene
[0207] Dissolve 130.1g of 2,3-difluorophenol and 234.0g of iodized ethane in 650mL of acetone, add 207.3g of anhydrous potassium carbonate, reflux for 2 hours, and cool to room temperature. After filtering and concentrating the filtrate, 600 mL of hexane was added to the residue, and the organic layer was washed successively with water and saturated brine. After drying over anhydrous sodium sulfate, it was concentrated to obtain 190 g of a reaction mixture. By distillation under reduced pressure, 134.1 g of 2,3-difluoroethoxybenzene was obtained as an oily substance. The boiling point is 95~96℃ / 57hPa.
[0208] (1-1-2) Synthesis of 2,3-difluoro-4-ethoxyphenol
[0209] 122.2 g of 2,3-difluoroethoxybenzene was dissolve...
Embodiment 2
[0233] (Embodiment 2) Synthesis of 1-butoxy-2,3-difluoro-4-(trans-4-vinylcyclohexyl)methoxybenzene (IIa)
[0234] (2-1) Synthesis of 2,3-difluoro-4-butoxyphenol
[0235] [chem 36]
[0236]
[0237] 2,3-difluoro-4-butane was synthesized in the same manner as the synthesis of 2,3-difluoro-4-ethoxyphenol described in Example 1 except for using iodized butane instead of iodized ethane Oxyphenol.
[0238] (2-2) Synthesis of 1-butoxy-2,3-difluoro-4-(trans-4-vinylcyclohexyl)methoxybenzene (IIa)
[0239] [chem 37]
[0240]
[0241] In the synthesis of 1-ethoxy-2,3-difluoro-4-(trans-4-vinylcyclohexyl)methoxybenzene (Ia), instead of 2,3-difluoro-4-ethoxy Using 2,3-difluoro-4-butoxyphenol instead of 2,3-difluoro-4-butoxyphenol, the same reaction was carried out to obtain 1-butoxy-2,3-difluoro-4-(trans-4 - vinylcyclohexyl)methoxybenzene (IIa).
[0242] MS m / z: 324 (M + ), 146(100)
[0243] 1 H-NMR (400MHz, CDCl 3 )
[0244] δ: 0.97(t, J=7.2 Hz, 3H), 1.00-1.25(m, 4H), 1.40...
Embodiment 3
[0245] (Embodiment 3) 4-ethoxy-2, the synthesis of 3-difluoro-1-(trans-4-(trans-4-vinylcyclohexyl)cyclohexyl)methoxybenzene (IIIa)
[0246] [chem 38]
[0247]
[0248] (3-1) Synthesis of 4,4'-dimethoxymethylene dicyclohexyl
[0249] Disperse 882.3 g of methoxymethyltriphenylphosphonium chloride in 2600 mL of THF and cool to -10°C. While maintaining the internal temperature, 313.2 g of potassium tert-butoxide was added. After stirring for 1 hour while maintaining the internal temperature, a THF (800 mL) solution of 200.0 g of dicyclohexyl-4,4'-dione was added dropwise. After stirring for 1 hour while maintaining the internal temperature, water was added to stop the reaction. After distilling off the solvent under reduced pressure, hexane was added and vigorously stirred, followed by filtration (twice). The filtrates were combined, washed successively with 50% methanol aqueous solution and saturated brine, and dried over anhydrous magnesium sulfate. The solvent was disti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com