Method for large-batch separating preparation of high-purity theaflavine monomer
A large-scale separation and high-purity technology, applied in organic chemistry and other directions, can solve the problems of poor separation selectivity, difficult purification, and difficulty in the separation of high-purity theaflavin monomers in large quantities. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
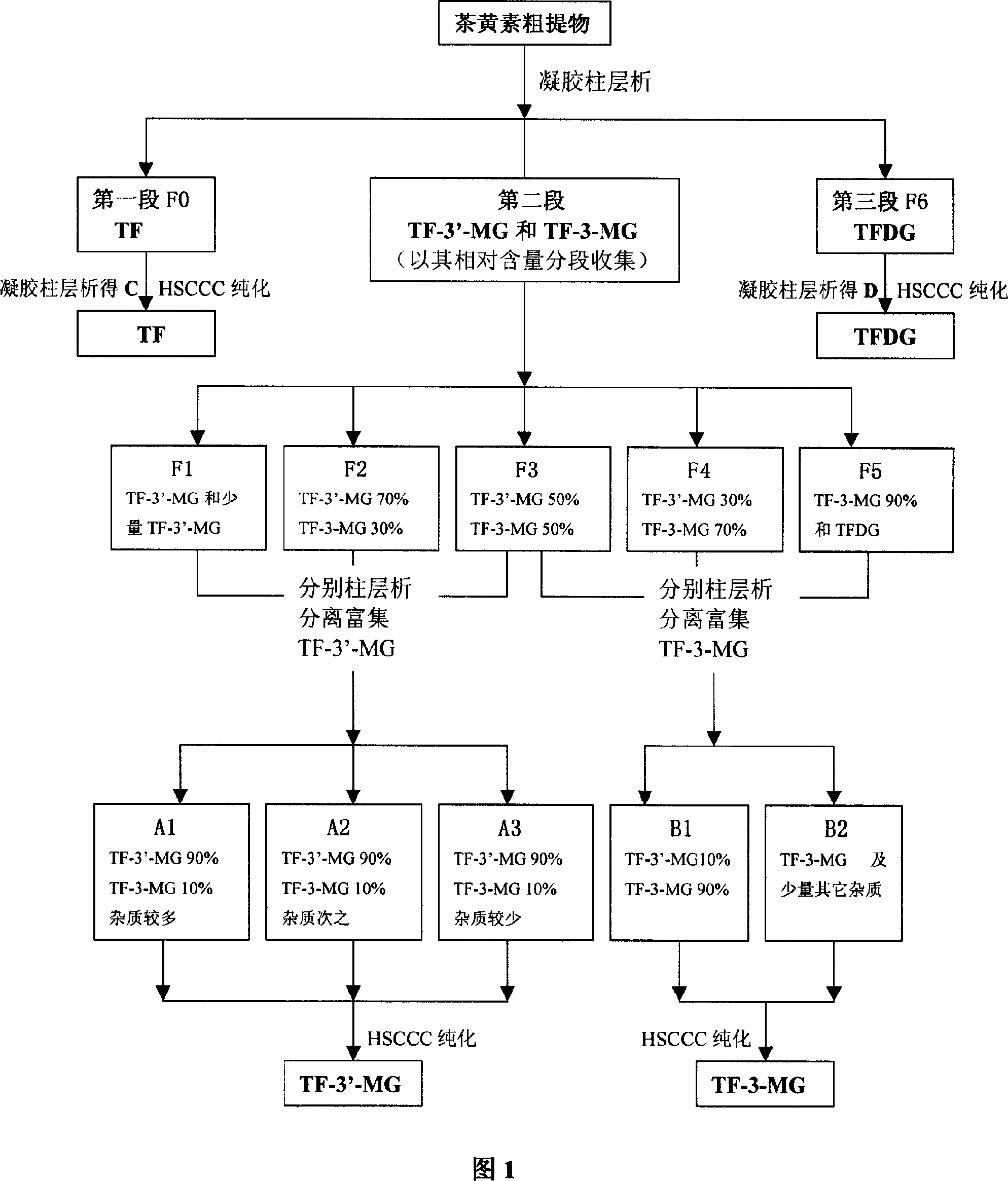
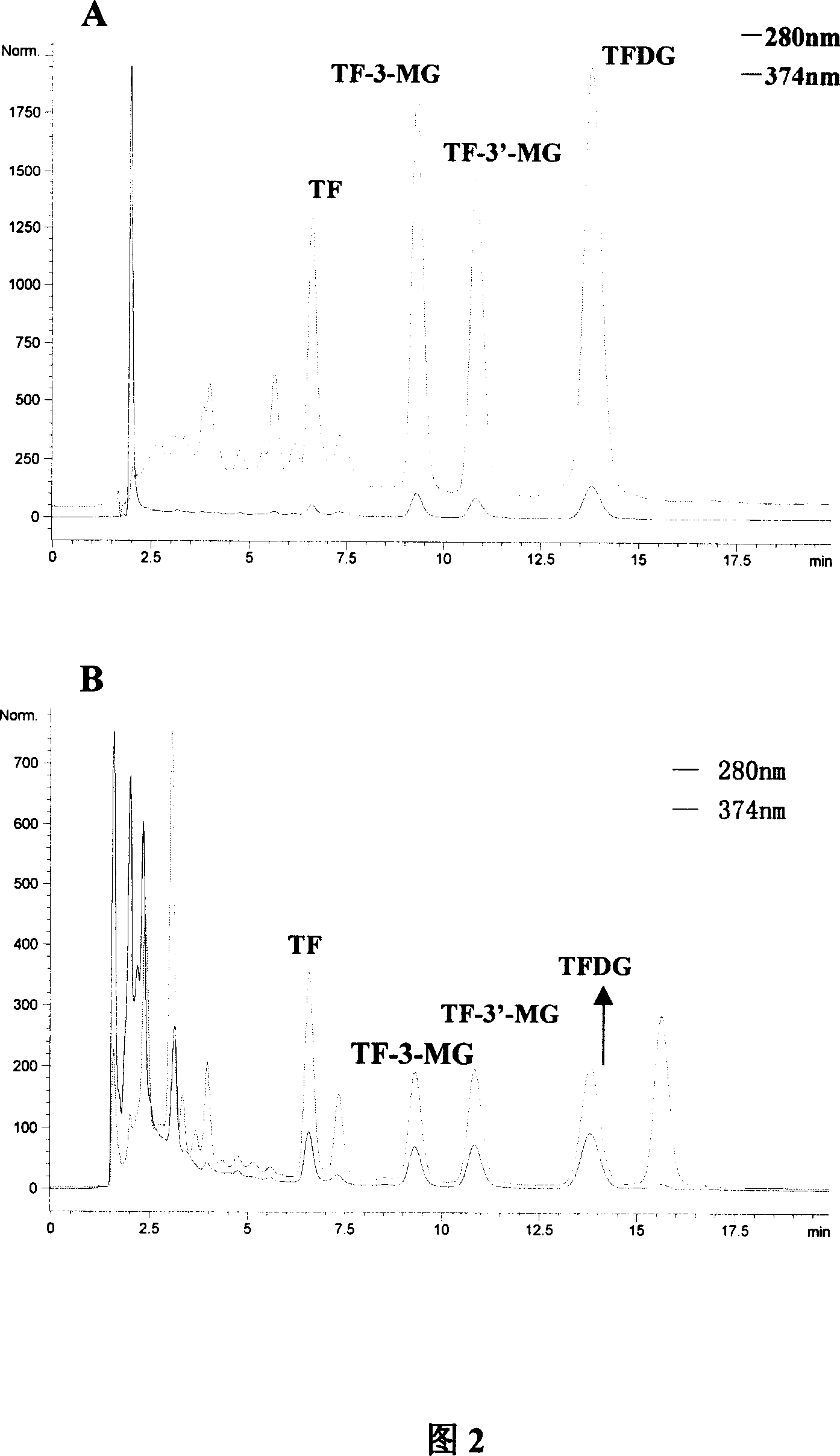
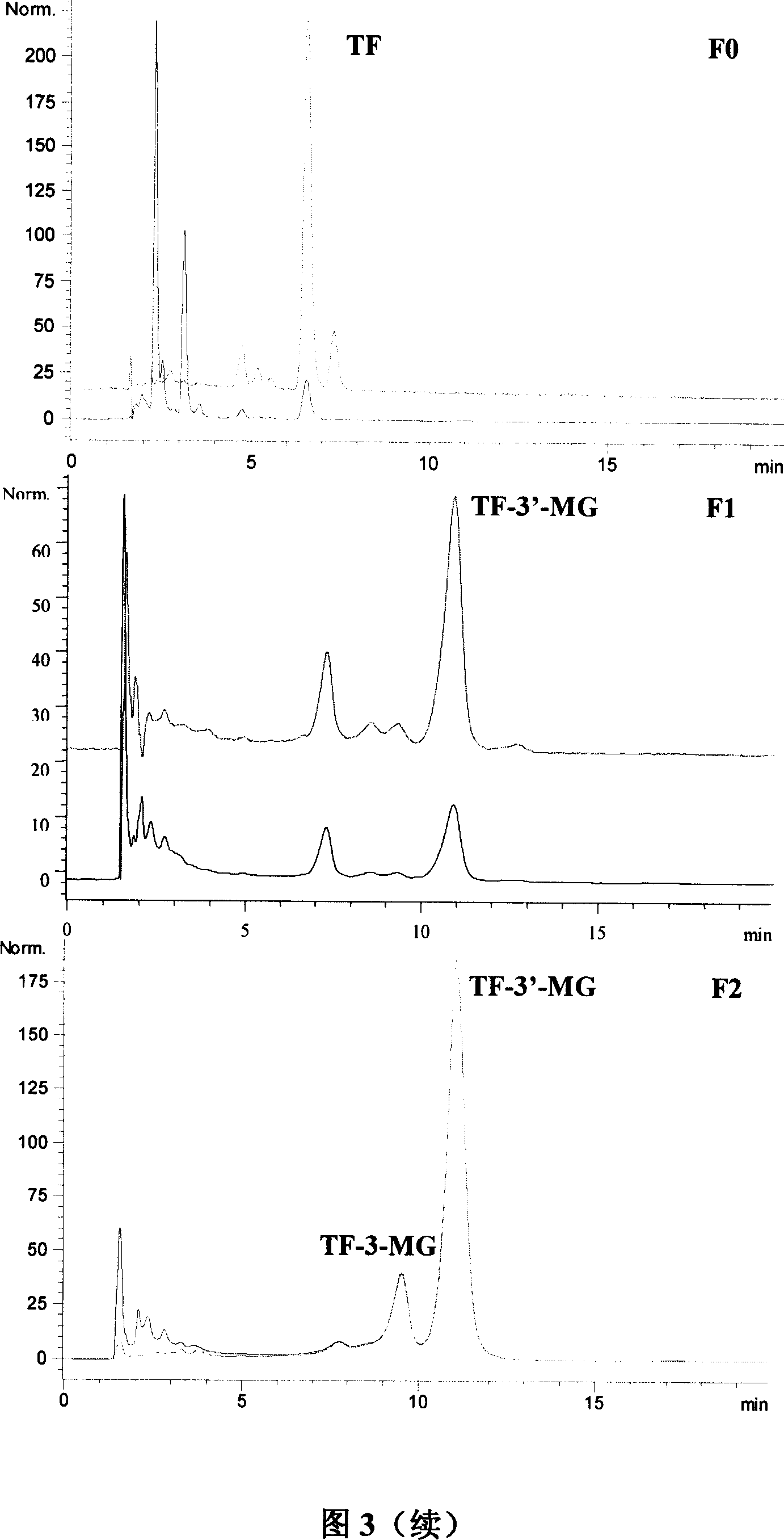
[0035] Example 1: The first step of column chromatography separation of theaflavin crude extract (referring to the flow chart of Fig. 1) takes black tea crude extract 40g, after dissolving with 70ml eluent, put on gel chromatographic column 1 (Sephadex LH- 20,900g, 100×10cm i.d.) for separation. Absolute ethanol was used as the eluent, and the average flow rate was 18ml / min. According to the color segment on the gel column after sample loading, collect in segments. After HPLC detection, the collected fractions were combined into three sections and 7 fractions, namely F0 mainly containing TF, F1, F2, F3, F4 containing TF-3-MG and TF-3'-MG, F5 (in order of elution), and the F6 fraction mainly containing TFDG. Figure 2 shows the HPLC analysis spectra of the Sigma mixed standard sample and the crude extract of theaflavins at two wavelengths. Analytical conditions: chromatographic column: Waters Xterra RP-C18 column (5 μ m, 150 * 4.6mm i.d.); Mobile phase: acetonitrile-water (28...
Embodiment 2
[0036] Example 2: Further isolation and purification of TF-3'-MG fraction
[0037] The TF-3'-MG relative content obtained by the separation of the crude sample is more than 50% F2, and the F3 fraction is put on a gel column for secondary separation respectively, and the column chromatography conditions adopted are the same as in Example 1. In the obtained fractions, the TF-3'-MG content in the 50%-90% fractions were separated by column chromatography for the third time. In this separation, 35%-acetone aqueous solution was used for elution. At this time, TF-3-MG The elution order of TF-3'-MG is opposite to the elution order of ethanol, TF-3-MG comes first, and TF-3'-MG comes after, so that more pure TF- 3'-MG. Three fractions A1 (7.83g), A2 (7.89g) and A3 (6.93g) with a relative content of TF-3'-MG obtained by repeated column chromatography separation and enrichment of more than 90% (wherein FL grade Fractions were combined with similar fractions) were further purified with H...
Embodiment 3
[0040] Example 3: Further isolation and purification of the TF-3-MG fraction
[0041] Combine the F4 and F5 with a relative content of TF-3-MG above 50% obtained from the separation of the crude sample and the similar fractions after the above-mentioned separation of TF-3'-MG. Fractions with a relative content of TF-3-MG of 50%-90% were separated by column chromatography for the third time, and the column chromatography conditions used were the same as those in Example 1. Grades B1 (0.52g) and B2 (17.27g) obtained by repeated separation and enrichment with a relative content of TF-3-MG above 90% were further purified by HSCCC to remove polar impurities therein.
[0042] Through HPLC analysis (as shown in Figure 7), it can be seen that B1 contains about 90% TF-3-MG and 10% TF-3'-MG; while B2 mainly contains TF-3-MG, TF- The 3'-MG content is very low, but contains a large amount of polar impurities in every fraction. Figure 8 shows the HSCCC separation spectrum of the B2 fract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com