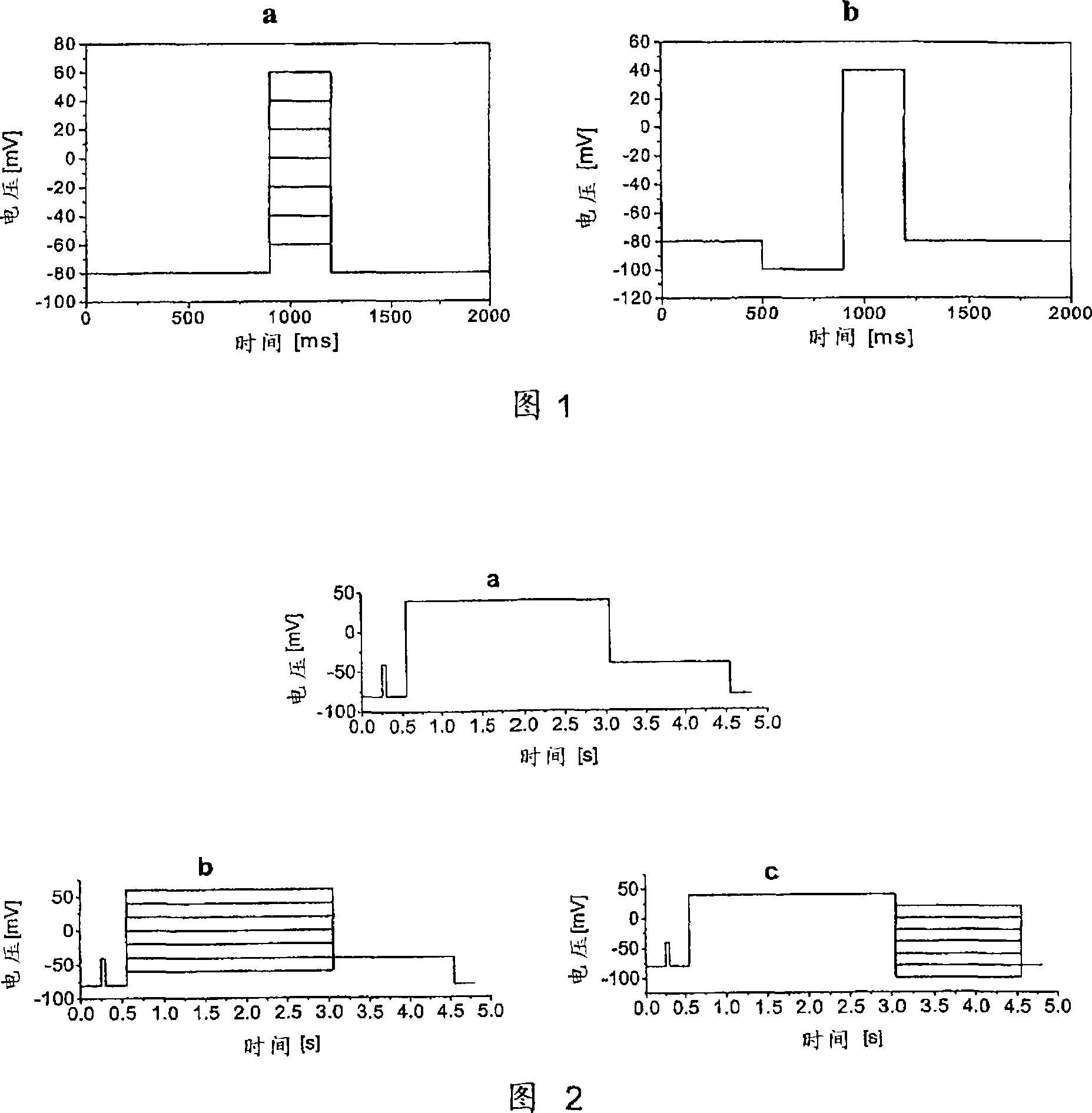
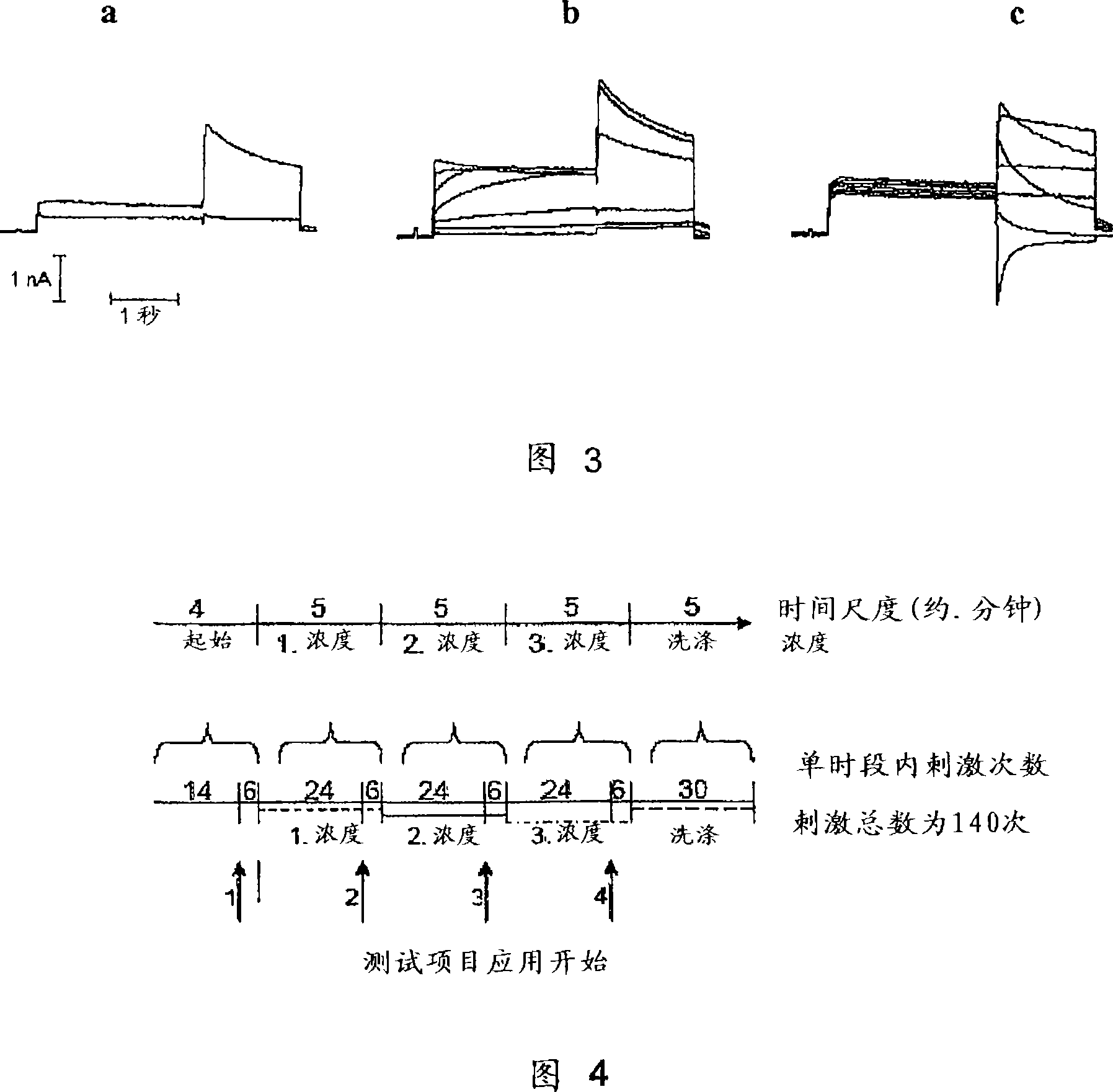
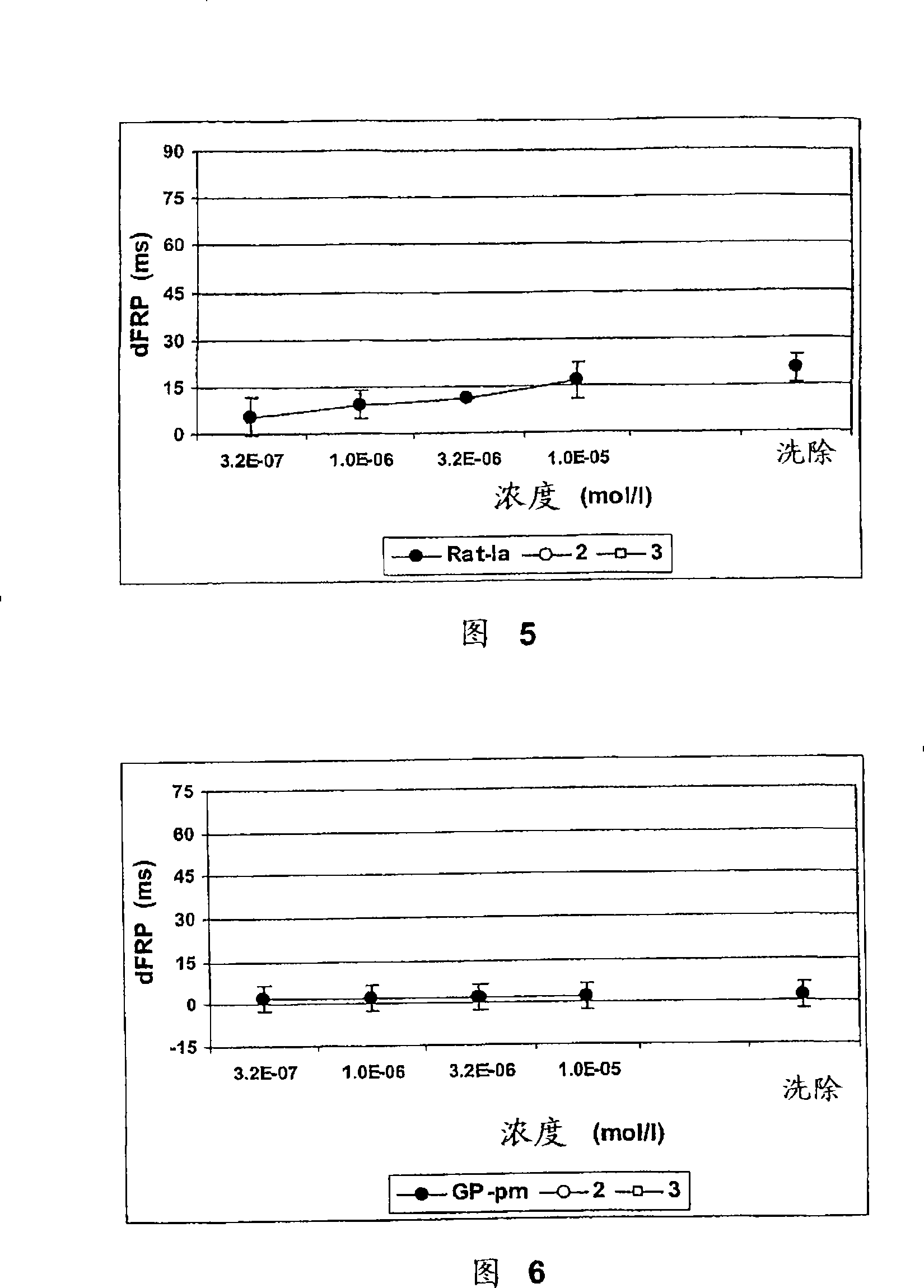
Compounds with Kv4 ion channel activity
A compound and metabolite technology, applied in the field of preparing the pharmaceutical composition and preparing the compound, can solve the problem of not being able to use the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0247] Example 1: Preparation of compounds according to the invention
[0248] The practice of the present invention will employ, unless otherwise indicated, conventional techniques in the fields of synthetic organic chemistry, biological testing and the like, which are within the skill of the art. Such techniques are explained fully in the literature. Purity of compounds was confirmed by liquid chromatography / mass spectrometry (LC / MS) according to Method A, unless otherwise indicated:
[0249] Method A:
[0250] HPLC: Waters Alliance 2690 with Photodiode Array Detector Waters 996. Mass spectrometer: Micromass Platform ZMD LC. Ionization: Electrospray (polarity: negative and positive).
[0251] method:
[0252] Phase: Tosohaas TSK-gel super ODS ( , 2μm), column: 4.6 × 50mm; Solvent A: water and formic acid (26.5mM); Solvent B: acetonitrile and formic acid (17mM); Flow rate: 2.75ml / min; Gradient 5min: from 100%A and 0 in 3 minutes %B to 20%A and 80%B. Constant 80% B fo...
Embodiment 2
[0306] Example 2: Non-limiting examples of compounds according to the invention
[0307] The present invention includes compounds of formulas I to LVII and stereoisomers, tautomers, racemates, prodrugs, metabolites, or pharmaceutically acceptable salts and / or solvates thereof.
[0308] Compound 1: 5-Chloro-benzofuran-2-carboxylic acid (R)-[(4-nitrophen-1-yl)ethyl]-amide
[0309] This compound was obtained according to Protocol A from 5-chloro-benzofuran-2-carboxylic acid and (R)-(4-nitrophen-1-yl)ethanamine.
[0310] Compound 2: 5-chloro-benzofuran-2-carboxylic acid 3,5-dimethoxybenzyl-amide
[0311] The present compound was obtained according to Protocol A from 5-chloro-benzofuran-2-carboxylic acid and 3,5-dimethoxybenzylamine.
[0312] Compound 3: 5-Chloro-benzofuran-2-carboxylic acid 2-(5-methylindol-3-yl)-ethyl-amide
[0313] This compound was obtained according to Protocol A from 5-chloro-benzofuran-2-carboxylic acid and 2-(5-methylindol-3-yl)-ethylamine.
[0314] Com...
Embodiment 3
[0331] Example 3: Biological Assay Using Caenorhabditis elegans (C.e / egans) Screening
[0332] A C. elegans-based high-throughput screen for Kv4.3 modulators has been used to establish the in vivo SAR (structure-activity relationship: effect of chemical structure on biological activity) of compounds according to the invention for Kv4.3.
[0333] The test employed a stable transgenic C. elegans strain that functionally expresses human Kv4.3 in the pharynx and a visual selection GFP marker in the body wall muscle.
[0334] Methods describing the construction of transgenic C. elegans strains expressing human Kv4.3 are described in WO03 / 097682. Briefly, 5 ng / μl pGV8 plasmid (human Kv4.3), 20 ng / μl pDW2821 (GFP-tagged), and 40 ng / μl genomic C. elegans DNA were microinjected into the gonads of wild-type strain N2 to generate practical The strain UG1755. Transgenic animals were isolated and extrachromosomal arrays were integrated into the C. elegans genome. 50% of the cell lines t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| shear viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com