Technique for synthesizing levorotatory betaxolol hydrochloride
A technology for the synthesis of betaxolol hydrochloride, which is applied in the field of synthesis of L-betaxolol hydrochloride, can solve the problems of high operational skill requirements, long production cycle, and high price, and achieve simple process methods and three-waste emissions Small, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
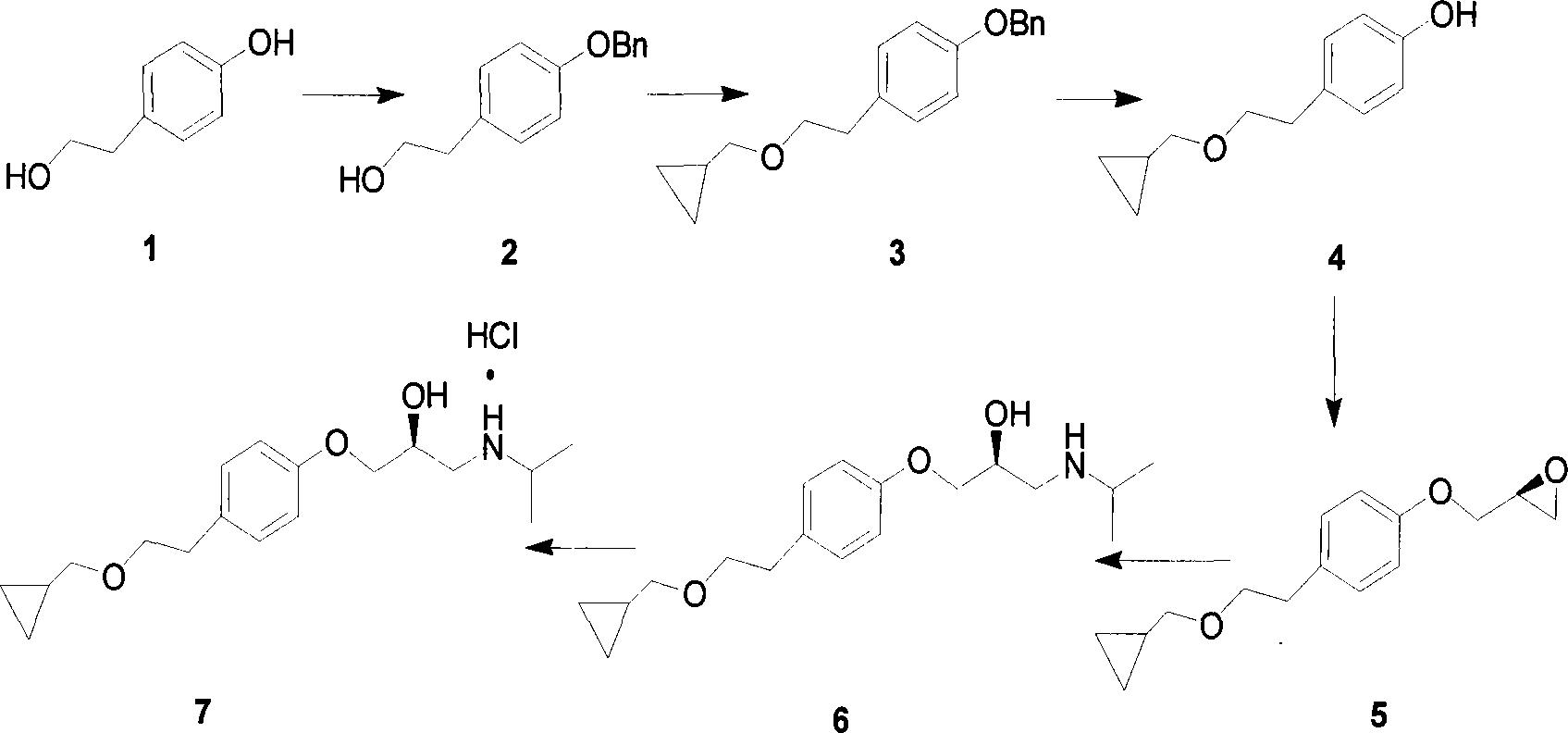
[0030] For a better description of the present invention, examples are as follows:
[0031] The preparation of embodiment L-betaxolol hydrochloride (compound 6)
[0032] (1) Preparation of Compound 2 (2-(4-benzyloxy)phenylethanol)
[0033] Dissolve p-hydroxyphenethyl alcohol (27.60g, 0.2mol) and benzyl chloride (50.40g, 0.4mol) in an organic solvent, then add sodium hydroxide (16.00g, 0.4mol) and stir mechanically, heat at 70°C for 10 Hour. After the reaction, the solid was removed by filtration, the filtrate was concentrated and evaporated to dryness, and water was added for recrystallization to obtain 44.23 g of 2-(4-benzyloxy)phenylethanol, with a yield of 97%. The experimental data are as follows:
[0034] C 15 h 16 o 2 , 1 HNMR (400MHz, CDCl 3 ): δ7.44-7.25(m, 5H), 7.14(d, 2H, J=8.5Hz), 6.93(d, 2H, J=8.5Hz), 5.05(s, 2H), 3.82(t, 2H, J=6.6Hz), 2.81(t, 2H, J=6.5Hz); 13 CNMR (100.6MHz, CDCl 3 ): δ157.51, 137.06, 130.67, 129.99, 128.58, 127.94, 127.45, 114.98, 70.02...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com