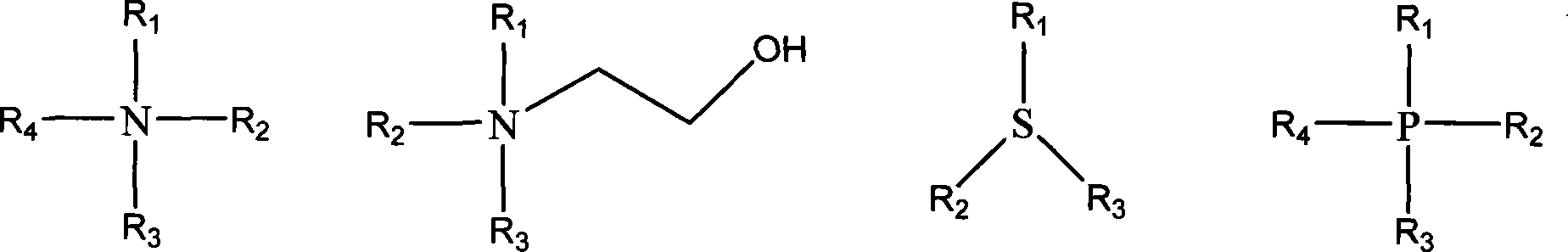
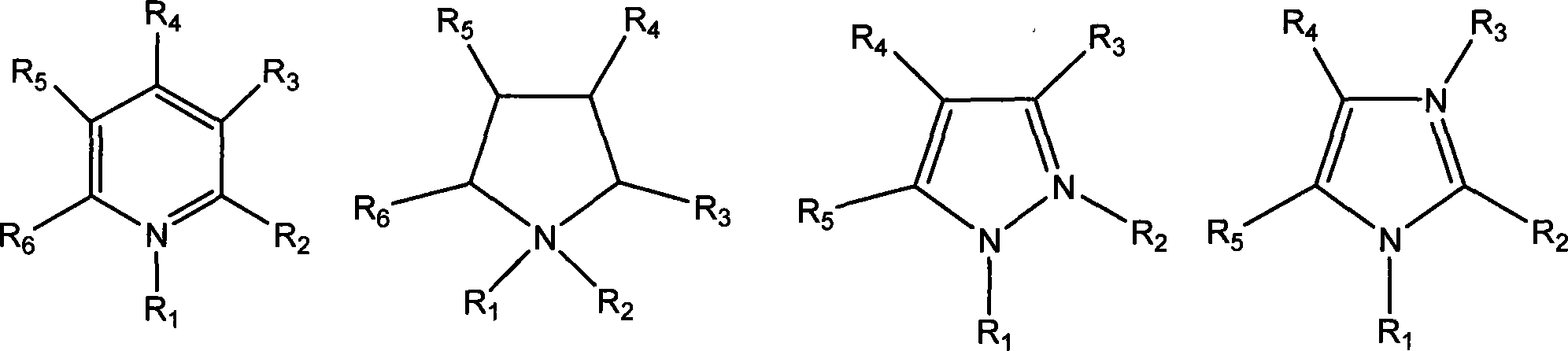
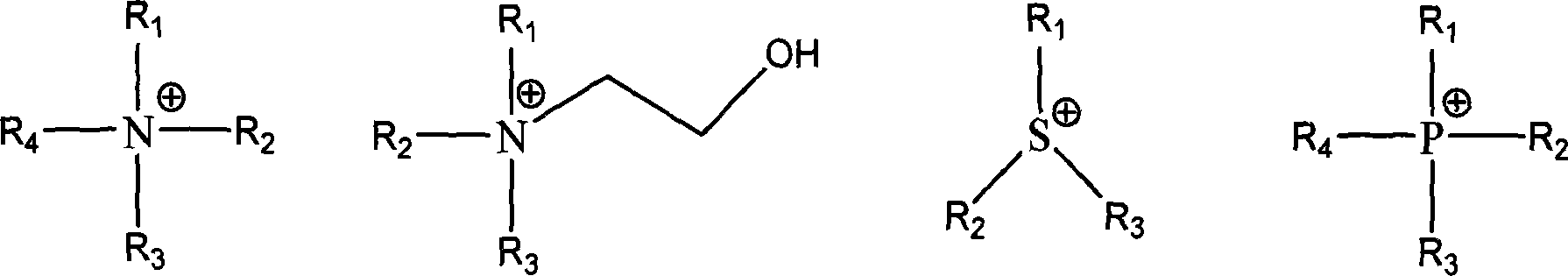
Method for preparing ionic liquid with anion being as ion in halogen family
A technology of ionic liquid and anion, applied in the field of ionic liquid preparation, can solve the problems of long reaction time, low conversion rate, affecting product quality, etc., achieve the effect of reducing the amount of solvent used, reducing recycling costs, and improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 41.055g (0.500mol) of N-methylimidazole and 46.300g (0.500mol) of n-chlorobutane successively in a 250ml pressurized and airtight reaction kettle, stir at room temperature to mix them uniformly and pass into N 2 After 5 minutes, heat the reaction kettle containing the reaction mixture to 85°C, control the pressure in the kettle to 0.12MPa, stop stirring after 8 hours of reaction, release the pressure in the kettle to normal pressure, and take out the reaction after the reactants are cooled to room temperature. The product was extracted 3 times with 20ml of ethyl acetate, and then distilled under reduced pressure at 80°C for 0.5h to obtain 85.695g of ionic liquid of 1-butyl 3-methylimidazolium chloride salt, the product calculated by N-methylimidazole The rate is 98.1%.
Embodiment 2
[0036] Add 41.055g (0.500mol) of N-methylimidazole and 38.648g (0.505mol) of allyl chloride successively in a 250ml pressurized and airtight reaction kettle, stir and add dropwise at room temperature to make it mix uniformly and pass into N 2 After 25 minutes, heat the reaction kettle containing the reaction mixture to 70°C, control the pressure inside the kettle to 0.16MPa, stop stirring after 0.5 hours of reaction, release the pressure inside the kettle to normal pressure, and take out the reaction after the reactants are cooled to room temperature. The product was extracted 3 times with 25ml of ethyl acetate, and then distilled under reduced pressure at 40°C for 0.8h to obtain 74.164g of ionic liquid of 1-allyl 3-methylimidazolium chloride salt, calculated as N-methylimidazole The yield was 93.5%.
Embodiment 3
[0038] Add 41.055g (0.500mol) N-methylimidazole and 68.515g (0.500mol) n-butane bromide successively in a 250ml pressurized and airtight reactor, stir at room temperature to make it evenly mixed and pass into N 2After 5 minutes, heat the reaction kettle containing the reaction mixture to 110°C, control the pressure in the kettle to 0.2MPa, stop stirring after 2 hours of reaction, release the pressure in the kettle to normal pressure, and take out the reaction after the reactants are cooled to room temperature. The product was extracted 3 times with 56ml of ethyl acetate, and then distilled under reduced pressure at 75°C for 1.2h to obtain 103.763g of ionic liquid of 1-butyl 3-methylimidazolium bromide. The rate is 94.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com