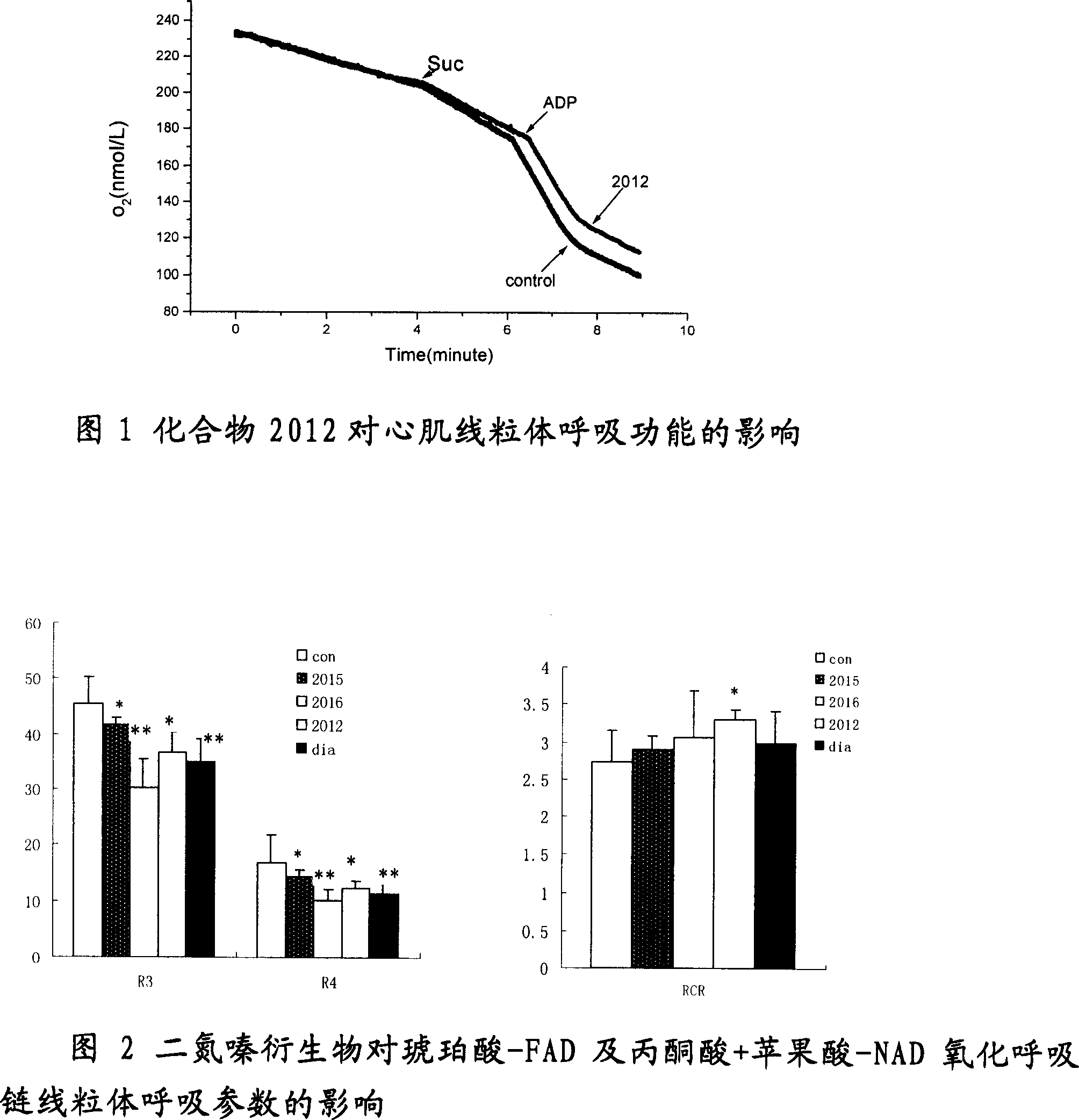
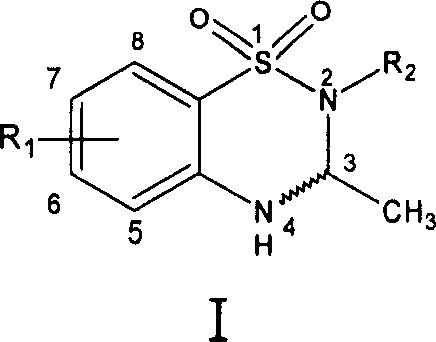
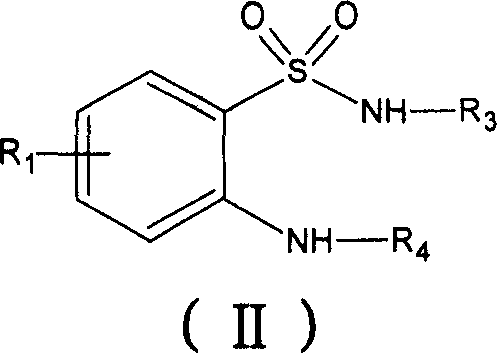
Derivative of new benzothiadiazines, preparation method and usage
A benzothiadiazine and a technology for its application are applied in the field of benzothiadiazine compounds and their preparation, and can solve the problems of elevated blood sugar, low tissue selectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Preparation N-{[2-(acetamide) phenyl] sulfonyl} acetamide
[0050] Add 34.4g of anthranilamide, 70ml of acetic anhydride, and 35ml of pyridine into a 250ml three-necked reaction flask, stir and heat, and react at 40°C to 100°C for 3 hours. The solution is reddish brown. During the reaction The material gradually precipitated again, cooled to below 0°C, filtered, rinsed with cold ethanol three times, and recrystallized from ethanol to obtain 43 g of N-{[2-(acetamide)phenyl]sulfonyl}acetamide. Melting point: 195.6-195.9°C, yield 84.0%.
Embodiment 2
[0051] Embodiment 2: Preparation of N-{[2-(acetamide)-4-chlorophenyl]sulfonyl}acetamide
[0052] Get 41.3g 4-chloro-2-aminobenzenesulfonamide, the acetic anhydride of 70ml, the pyridine of 35ml, preparation method is the same as embodiment 1, obtains N-{[2-(acetamide)-4-chlorophenyl]sulfonyl } Acetamide 51g. Melting point: 217.1-218.2°C, yield: 81.7%.
Embodiment 3
[0053] Example 3: Preparation of 7-chloro-2,3-methyl-2H-1,2,4-benzothiadiazine-1,1-diox (compound code 2001)
[0054] Take 7-chloro-3-methyl-2H-1,2,4-benzothiadiazine-1,1-dioxygen 7g, anhydrous potassium carbonate 12.5g, acetonitrile 80ml, add it into a 250ml three-necked reaction flask, Add dropwise the mixed solution of 13.7g methyl iodide and 10ml acetonitrile under stirring, raise the temperature, keep the temperature at about 55°C, react for 3 hours, recover the solvent under reduced pressure, pour the reaction solution into 200ml water, stir for 10 minutes, filter, A khaki solid was obtained, which was recrystallized from 200 ml of ethanol to obtain 6.5 g of light yellow solid 7-chloro-2,3-methyl-2H-1,2,4-benzothiadiazine-1,1-dioxine. Melting point: 235.7-236.2°C, yield 83.3%.
[0055] Prepare the following compounds in the same way:
[0056] 7-Chloro-3-methyl-2-ethyl-2H-1,2,4-benzothiadiazine-1,1-dioxo, melting point: 200.6-201.1°C, yield 65.0%. (Compound code 2002) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com