Use of erythropoietin
A technology of erythropoietin and endothelial cells, which is applied in the application field of erythropoietin, and can solve problems such as the mobilization and differentiation of endothelial progenitor cells that have not been fully understood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Effect of EPO on Patients with Renal Anemia
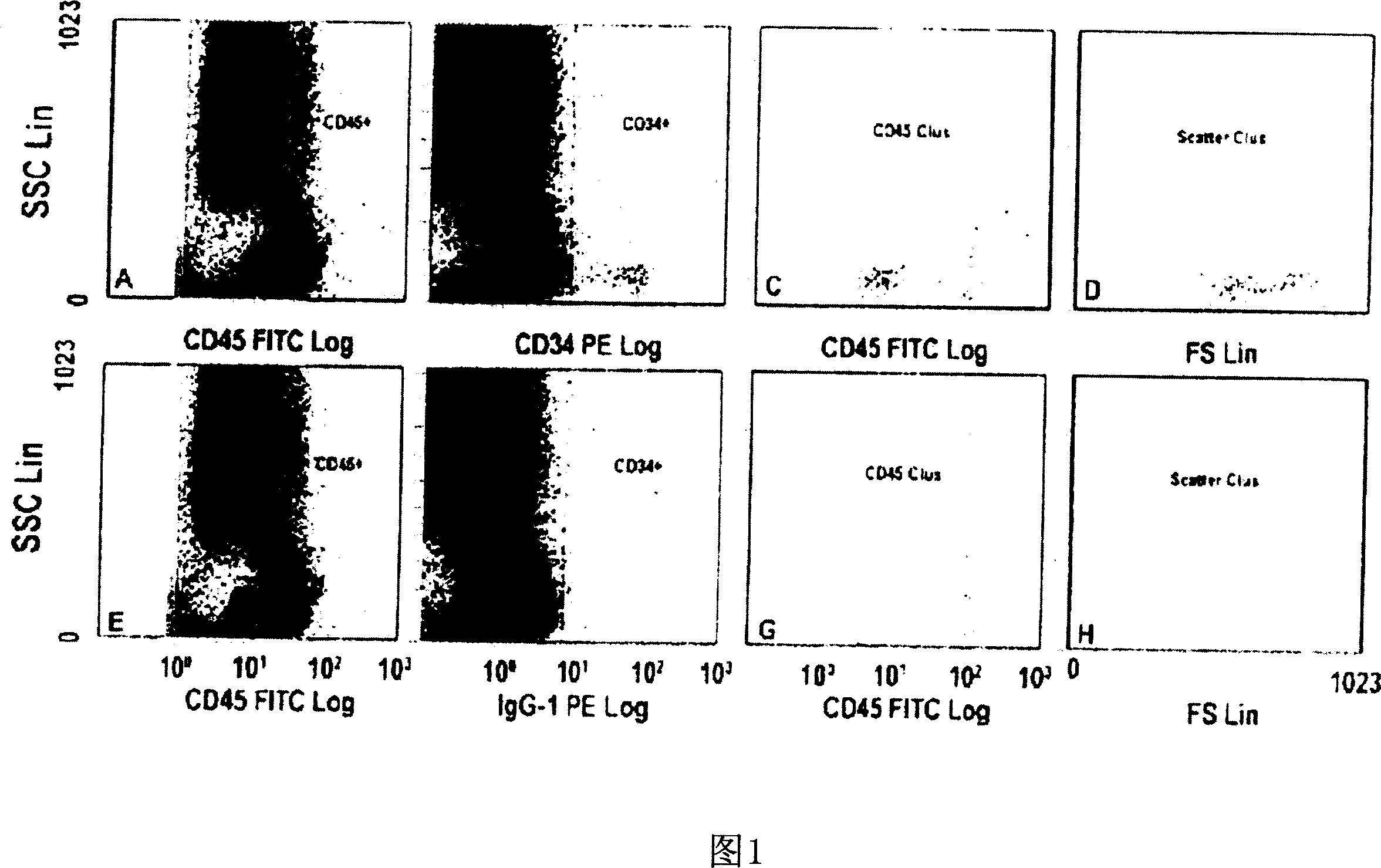
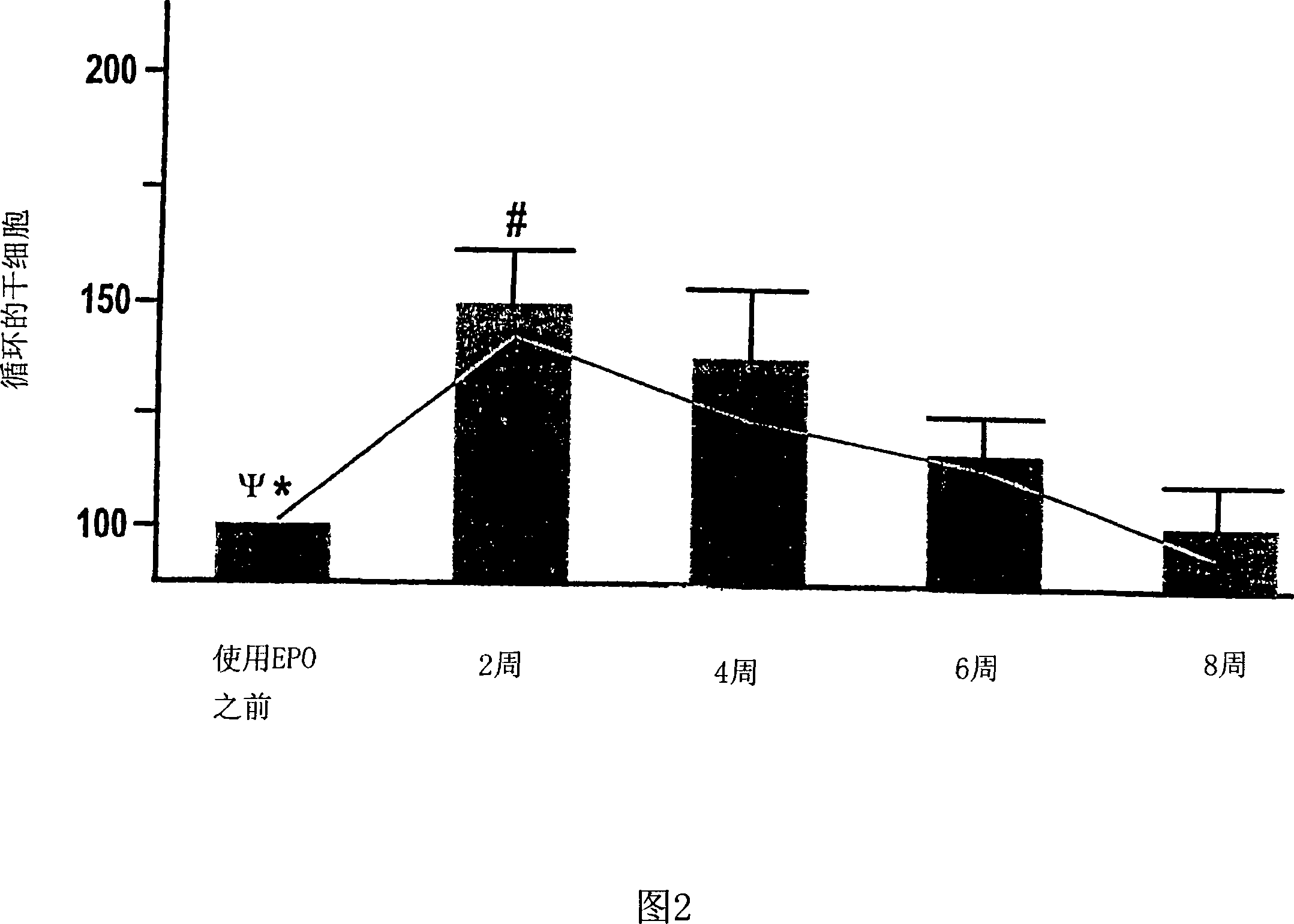
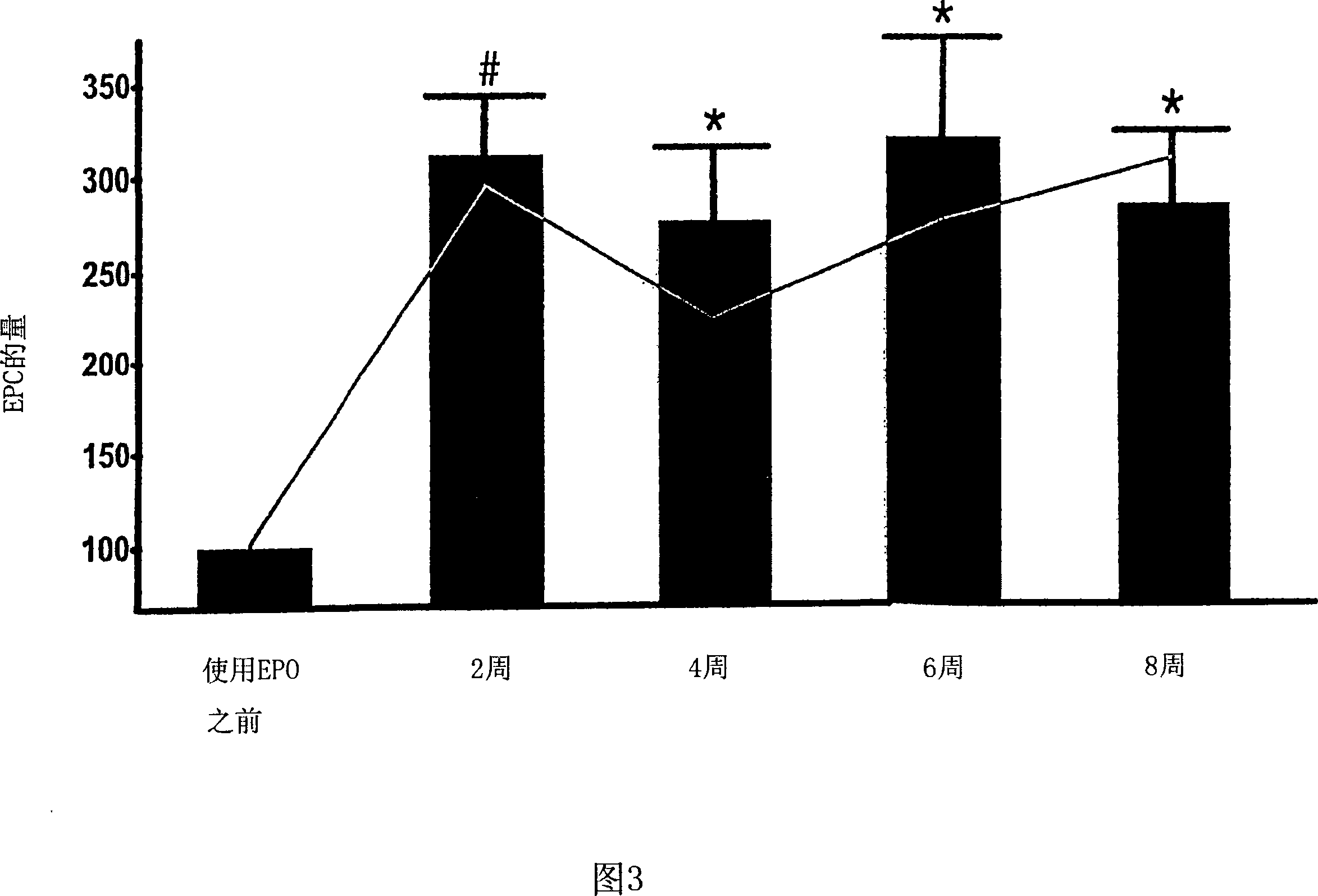
[0118] The role of EPO in patients with renal anemia (Hb < 10.5 g / dl) was investigated. Wherein, the renal anemia is an inevitable result of the late stage of renal disease (the final stage of renal failure, creatinine clearance <35ml / min). According to the average dose of 5000IU per week, 11 patients were treated with rhEPO (recombinant human erythropoietin) by intravenous injection or subcutaneous injection for at least 8 weeks. Endothelial progenitor cells in the patient's blood were studied within 20 weeks after erythropoietin treatment, during which time, 0, 2, 4, 6 and 8 weeks later, by flow cytometry The number and differentiation state of endothelial progenitor cells were analyzed by counting method and culture analysis method.
[0119] Circulating peripheral blood stem cells (CPBSCs) include a small population of cells that express both CD34 and CD45 antigens. On the basis of the ISHAGE guidelines (Sutherland et ...
Embodiment 2
[0127] Improved wound healing through systemic use of rhEPO
[0128] FVB / N- mice were anesthetized by inhalation of isoflurane (Isoflorane). Remove the hair on the two hind legs with a depilatory solution and disinfect with 70% alcohol. Then, a sterile 4mm disposable biopsy drill was used to create skin wounds on the right side of these mice, and the opposite side was used as a control. A one-time antibiotic treatment was performed with penicillin G (20000 units / kg) after the operation. The recombinant human erythropoietin mimetic Aranesp (0.1 μg / kg body weight) was injected subcutaneously once a week throughout the experimental study period. Treatment begins seven days before the biopsy is removed. As shown in Figure 5, the results showed that the use of EPO accelerated the wound healing process quite significantly.
Embodiment 3
[0130] Prevention of the development of chronic renal failure by erythropoietin therapy
[0131] Eight week old Spraque-Dawley rats were anesthetized with ketamine (120 mg / kg) and xylazine (10 mg / kg). Right kidneys from these mice were removed on day 0 and immediately fixed in formalin for histological studies. The segmental arteries supplying blood to the upper and lower poles of the left kidney were ligated. This leads to renal infarction in the corresponding renal area, while only the middle third of the renal area remains functional. Once a week, these mice were injected subcutaneously with the erythropoietin analog Aranesp at a dose of 0.1 μg / kg body weight, or NaCl as a control group.
[0132] Figure 7 shows the Kaplan-Mayer survival curves for the two experimental groups. Mice treated with Aranesp had significantly improved survival compared to saline-treated control mice.
[0133] Figures 12-15 show that after treatment with erythropoietin, the kidney tissue did no...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com