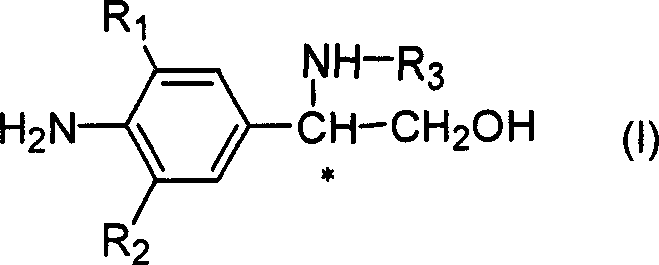
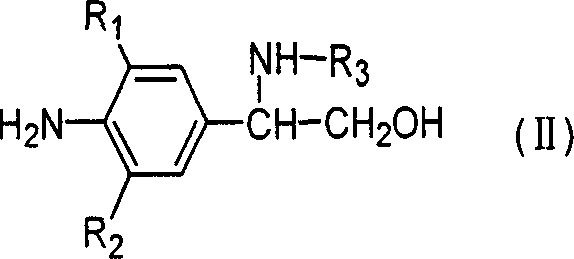
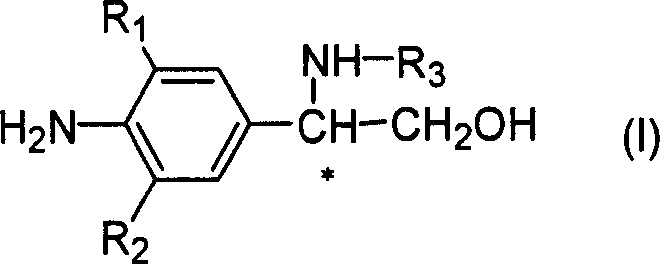
Novel optical activity phenylethanolamine compounds and preparation method thereof
A compound, the technology of tert-butylaminoethanol, which is applied in the field of phenylethanolamine compounds, can solve the problem that there is no description of the optical activity of phenylethanolamine compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (-)-2-(3-chloro-4-amino-5-trifluoromethylphenyl)-2-tert-butylaminoethanol hydrochloride and (+)-2-(3-chloro-4-amino-5 -Trifluoromethylphenyl)-2-tert-butylaminoethanol hydrochloride
[0037] a) (-)-2-(3-Chloro-4-amino-5-trifluoromethylphenyl)-2-tert-butylaminoethanol hydrochloride
[0038] Dissolve 4.45g (0.0143mol) of 2-(3-chloro-4-amino-5-trifluoromethylphenyl)-2-tert-butylaminoethanol in 53.4ml of absolute ethanol, drop 2.57g (0.00717mol) A solution prepared from dibenzoyl-D-tartaric acid and 25.7ml of absolute ethanol was added to 188ml of petroleum ether (bp.60-90°C), stirred for 1 hour, filtered, and dried to obtain 2-(3-chloro-4-amino -5-trifluoromethylphenyl)-2-tert-butylaminoethanol dibenzoyl-D-tartrate 2.9g, yield 82.6%.
[0039] Add 2.9g of 2-(3-chloro-4-amino-5-trifluoromethylphenyl)-2-tert-butylaminoethanol dibenzoyl-D-tartrate into 60ml of water, stir, and drop in 20% sodium hydroxide The solution was adjusted to pH=10, extracted with ether, and dried ov...
Embodiment 2
[0048] (-)-2-(3-trifluoromethyl-4-aminophenyl)-2-tert-butylaminoethanol hydrochloride and (+)-2-(3-trifluoromethyl-4-aminophenyl) -2-tert-butylaminoethanol hydrochloride
[0049] a) (-)-2-(3-trifluoromethyl-4-aminophenyl)-2-tert-butylaminoethanol hydrochloride
[0050] Dissolve 4.5g (0.0163mol) of 2-(3-trifluoromethyl-4-aminophenyl)-2-tert-butylaminoethanol in 65ml of isopropanol, add dropwise 1.23g (0.0082mol) of L-tartaric acid and 25ml The solution prepared in isopropanol was stirred for 2 hours, filtered, and dried to obtain 2.24 g of 2-(3-trifluoromethyl-4-aminophenyl)-2-tert-butylaminoethanol L-tartrate, with a yield of 78.1%.
[0051] Add 2.24g of 2-(3-trifluoromethyl-4-aminophenyl)-2-tert-butylaminoethanol L-tartrate into 50ml of water, stir, add dropwise 20% sodium hydroxide solution to pH=10, extract with ether, Dry over anhydrous sodium sulfate. Filtrate, evaporate ether under reduced pressure to obtain 1.67 g of (-)-2-(3-trifluoromethyl-4-aminophenyl)-2-tert-but...
Embodiment 3
[0058] (-)-2-(3-Chloro-4-amino-5-cyanophenyl)-2-tert-butylaminoethanol hydrochloride and d-2-(3-chloro-4-amino-5-cyanophenyl base)-2-tert-butylaminoethanol hydrochloride
[0059] a) (-)-2-(3-Chloro-4-amino-5-cyanophenyl)-2-tert-butylaminoethanol hydrochloride
[0060] Dissolve 2.5g (0.0093mol) of 2-(3-chloro-4-amino-5-cyanophenyl)-2-tert-butylaminoethanol in 50ml of absolute ethanol, and drop into 1.67g (0.0047mol) of diphenylmethane Acyl-D-tartaric acid and 16.7ml of absolute ethanol solution, add 200ml of anhydrous ether, stir for 1 hour, filter, and dry to obtain 2-(3-chloro-4-amino-5-cyanophenyl)- 1.58 g of 2-tert-butylaminoethanol dibenzoyl-D-tartrate, yield 75.6%.
[0061] Add 1.58g of 2-(3-chloro-4-amino-5-cyanophenyl)-2-tert-butylaminoethanol dibenzoyl-D-tartrate into 32ml of water, stir, and drop in 20% sodium hydroxide solution to pH=10, extracted with ether, dried over anhydrous sodium sulfate. Filtrate, evaporate ether under reduced pressure to obtain 0.9 g of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com