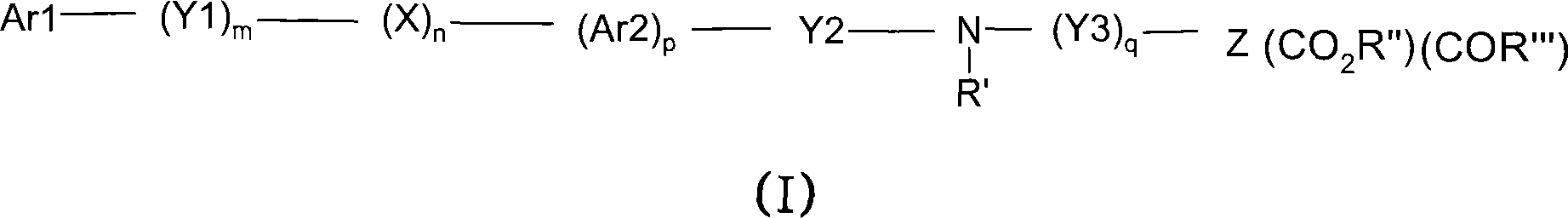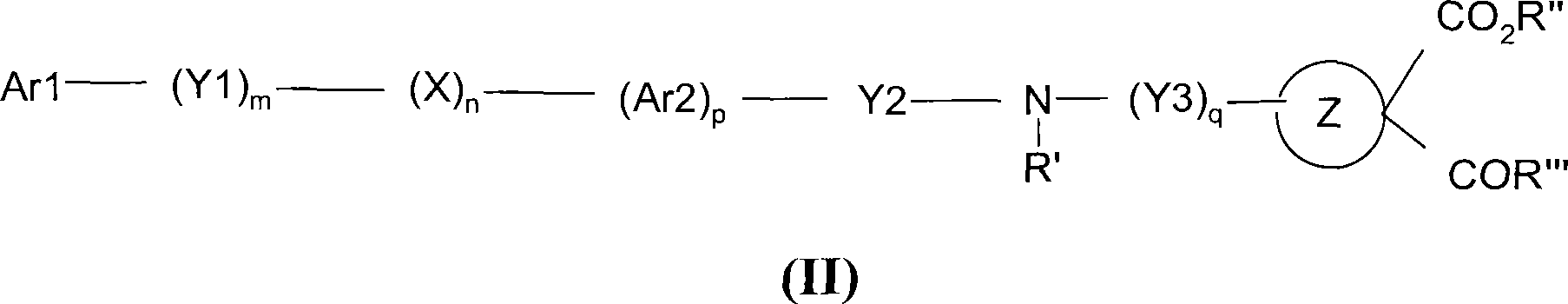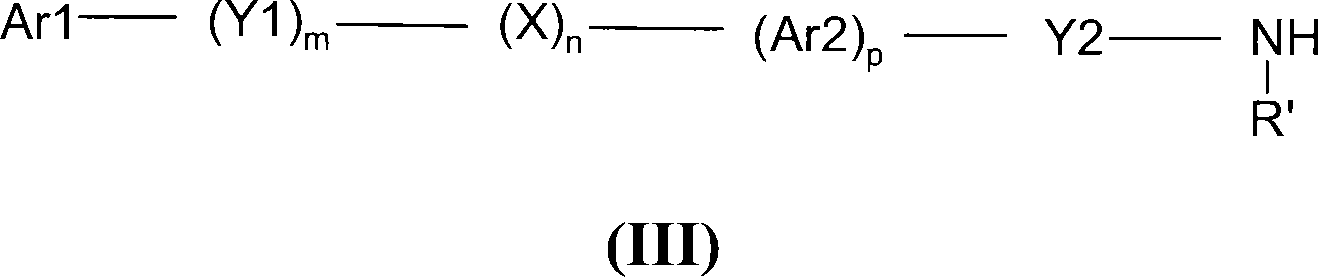Novel dicarboxylic acid derivatives
A compound, alkyl technology, applied in the field of new dicarboxylic acid derivatives, can solve problems such as lack of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0398] Preparation of C 4-nonylbenzylamine
[0399] Step I
[0400] synthetic route:
[0401]
[0402] Triethylsilane (20 mL) was added dropwise to a stirred solution of 1-(4-bromophenyl)nonan-1-one (10 g, 0.033 mol) in TFA (20 mL) at room temperature. The mixture was then heated to reflux for 4.5 h. The reaction mixture was cooled and concentrated under reduced pressure. The resulting residue was purified by column chromatography (silica gel 230-400 mesh, n-hexane:ethyl acetate; 95:5) to obtain 1-bromo-4-nonylbenzene.
[0403] Reference: J. Org. Chem. 38, 2675 (1973)
[0404] Step II
[0405] synthetic route:
[0406]
[0407] A small amount of iodine crystals was added to a heterogeneous solution of magnesium (0.771 g, 0.032 mol) in THF (10 mL) under a nitrogen atmosphere, followed by 5 mL of 1-bromo in THF (40 mL) at 650 C under vigorous stirring - 4-Nonylbenzene (6.5 g, 0.0229 mol). After starting, the remaining 1-bromo-4-nonylbenzene solution was added dropw...
Embodiment 1 and 2
[0445] 3-(4-Octylbenzylamino)cyclopentane-1,1-dicarboxylate hydrochloride and 3-(4-octylbenzylamino)cyclopentane-1,1-dicarboxylic acid ethyl ester
[0446] A solution of diethyl 3-oxocyclopentane-1,1-dicarboxylate (0.55 g, 0.00241 mol) in dichloromethane (5 mL) was added to stirred 4-octylbenzylamine ( 0.53 g, 0.00242 mol) in a solution in dichloromethane (20 mL) and methanol (2 mL). The reaction mixture was cooled to 5 to 100C and sodium cyanoborohydride (0.530 g, 0.0084 mol) was added. The reaction mixture was allowed to come to room temperature and stirred at room temperature for 4 h. The reaction mixture was concentrated under reduced pressure, and the residue was quenched with water (10 mL). Extracted in ethyl acetate (2 x 20 mL). The combined organic layers were washed with brine (1 x 10 mL) and dried over sodium sulfate. The solvent was removed to obtain a viscous liquid, which was purified by column chromatography (silica gel 230-400 mesh, mobile phase n-hexane:et...
Embodiment 3
[0454] Example 3 as hydrochloride
[0455] 13 C NMR: (CDCl3+TFA; 100.61MHz; ppm)
[0456] 14.66(q);23.32(t);29.48(t);29.97(t);29.98(t);30.10(t);30.18(t);31.86(t);32.55(t);33.59(t); 36.29(t);37.40(t);51.77(t);58.54(d);59.82(s);126.78(s);130.19(2d);130.28(2d);146.54(s);175.62(s); 176.89(s)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com