Compound isoglycyrdeuteroside and preparation and usage thereof
A technology of glycyrrhetin and crude glycyrrhetin, which is applied in the direction of steroidal compounds, food preparation, application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
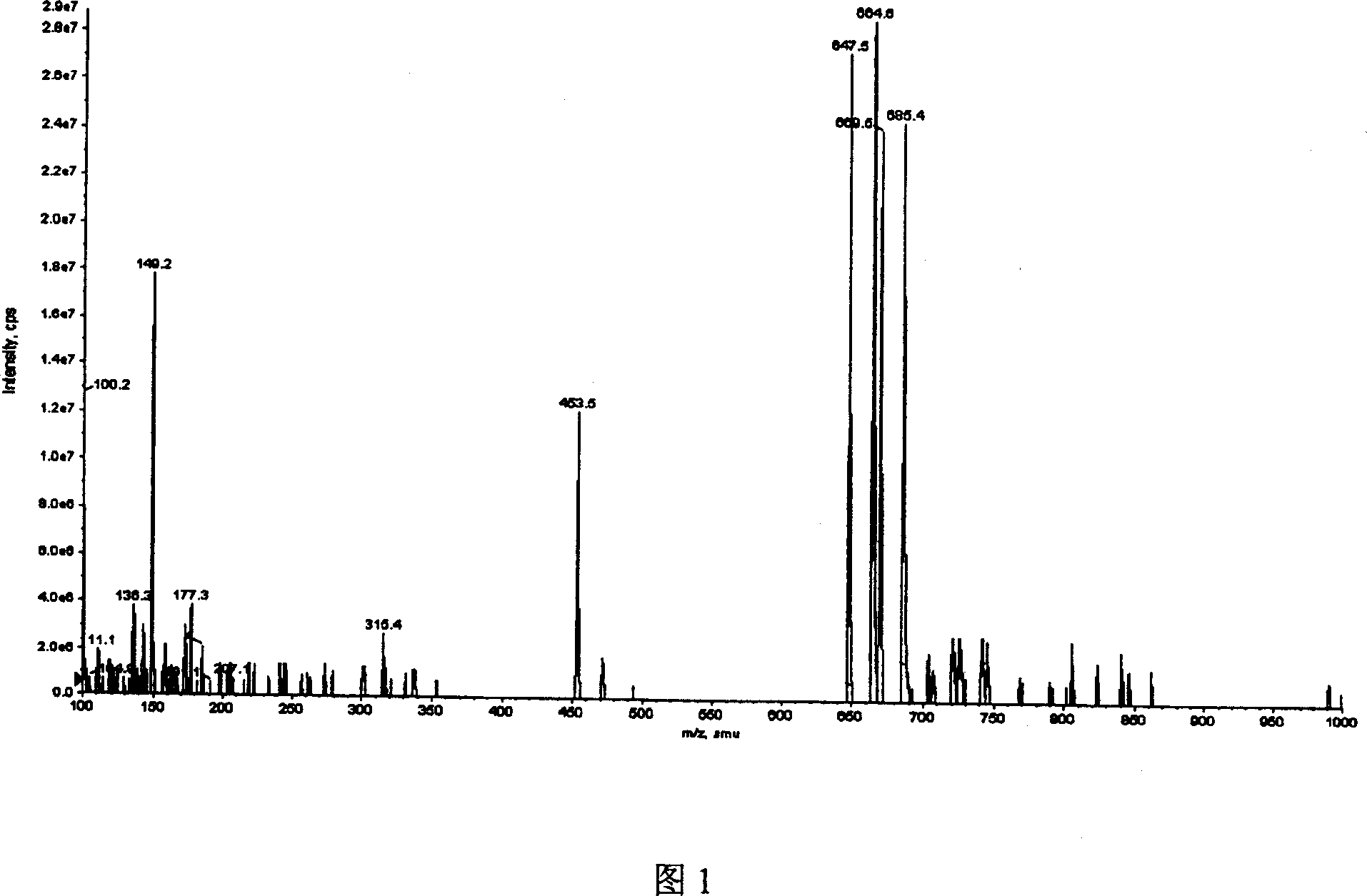
[0020] In a 50mL conical flask with a stopper, add 10mL of 20g / L 18α-glycyrrhizic acid solution (pH 6.0), then add 0.2g of pig liver enzyme powder (containing β-glucuronidase), and cover the grinding mouth tightly. Bottle stopper, react for 24h on a water bath shaker at 50°C and 120r / min. After the reaction was completed, the pH of the reaction solution was adjusted to 2-3, extracted with n-butanol for 2-3 times, the extracts were combined, and the organic solvent was removed by rotary evaporation to obtain 0.16 g of crude isoglycyrrhizin with a purity of 72% as determined by HPLC.
Embodiment 2
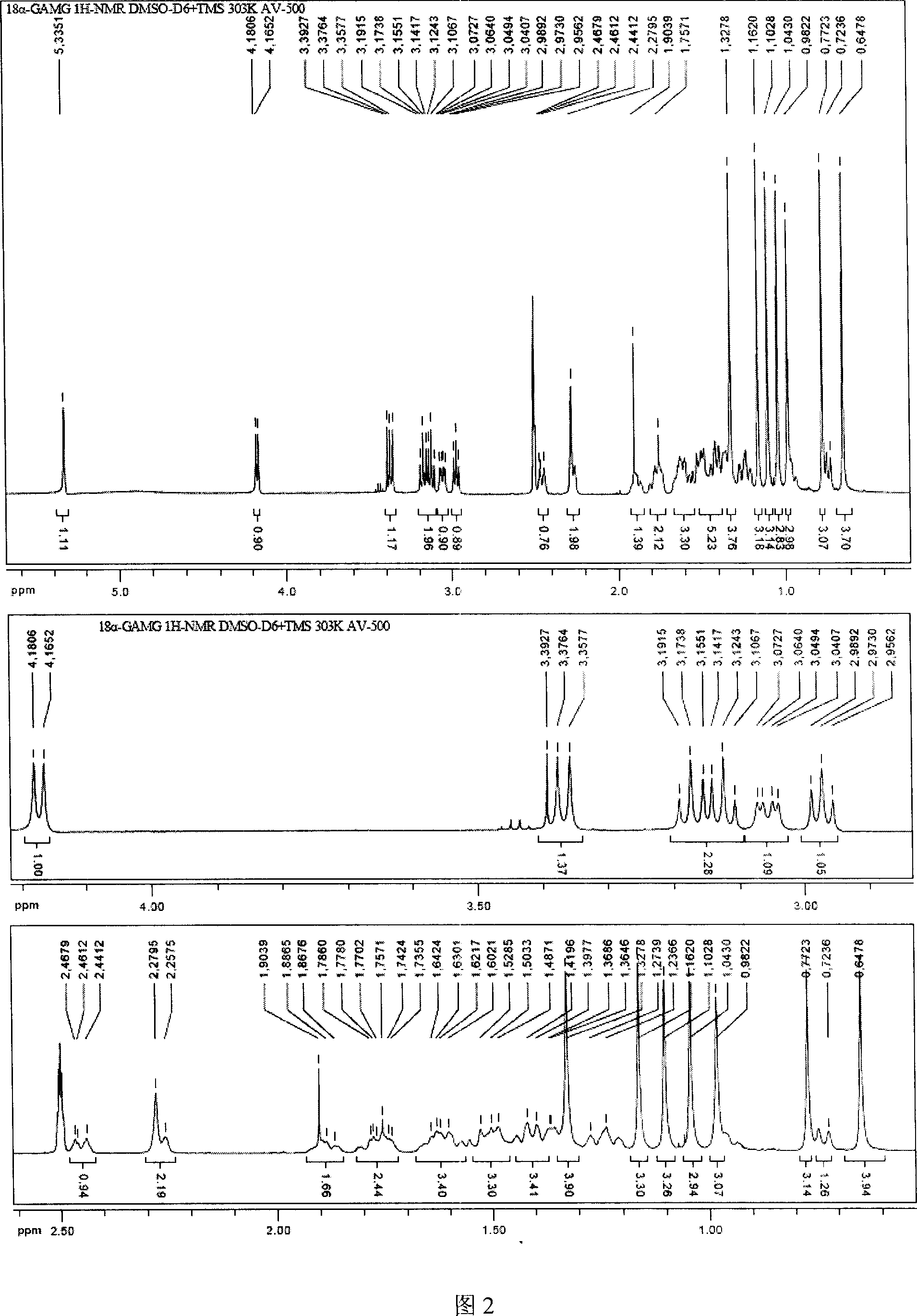
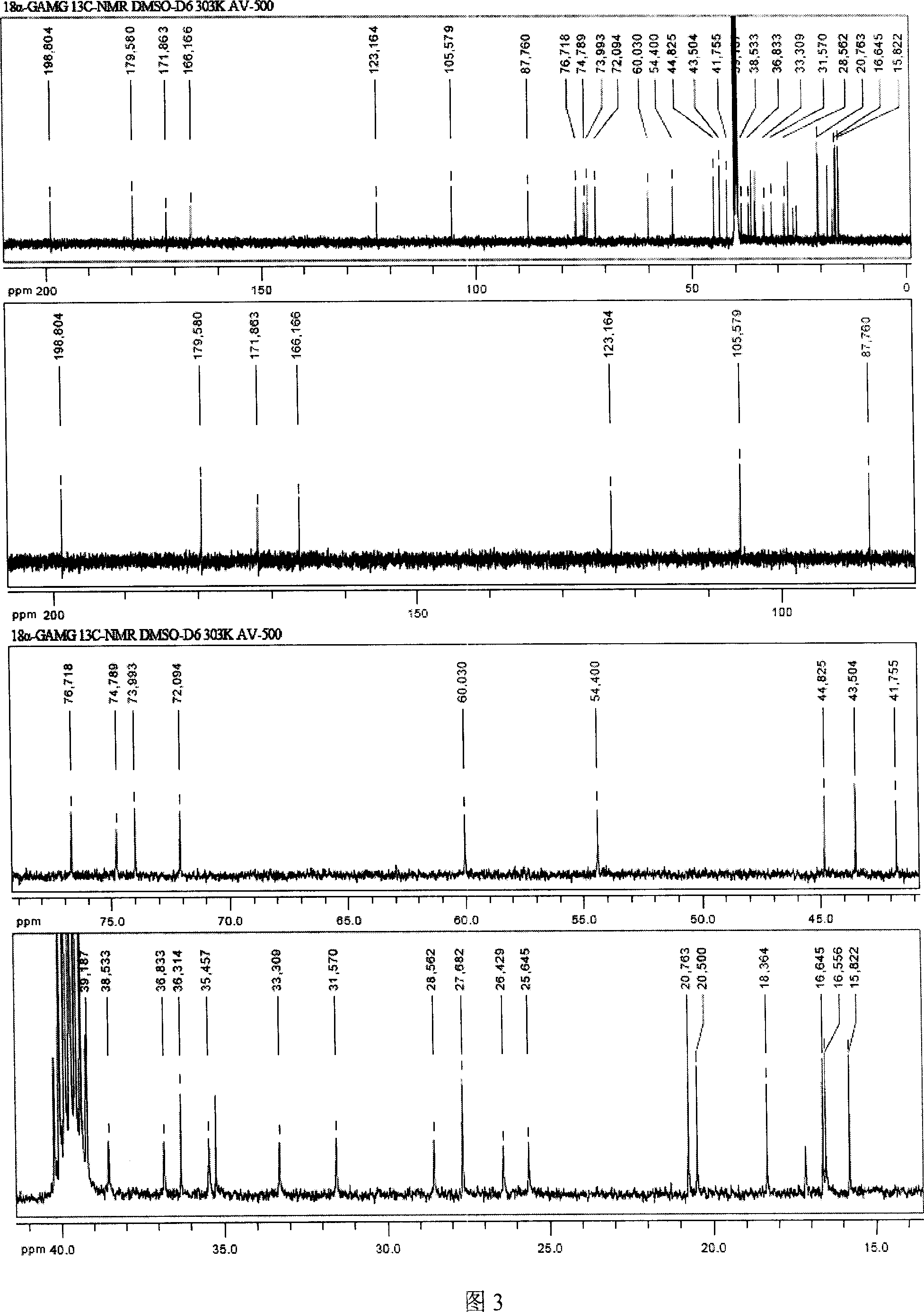
[0022] The crude isoliquiritiside prepared according to Example 1 was recrystallized 3-5 times with 85% glacial acetic acid to obtain 0.12 g of isoliquiritiside, the sweetness of which was about 900 times that of sucrose. That 1 H-NMR and 13 Assignment of C-NMR signals (DMSO-d 6 , internal standard TMS, 303K) as follows:
[0023] No.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com