Polysubstituted decahydronaphthalene compound, synthesis method and uses thereof
A compound, decalin technology, applied in the field of multi-substituted decalin compounds, can solve problems such as complex oxidative degradation, and achieve the effects of simple and easy method, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
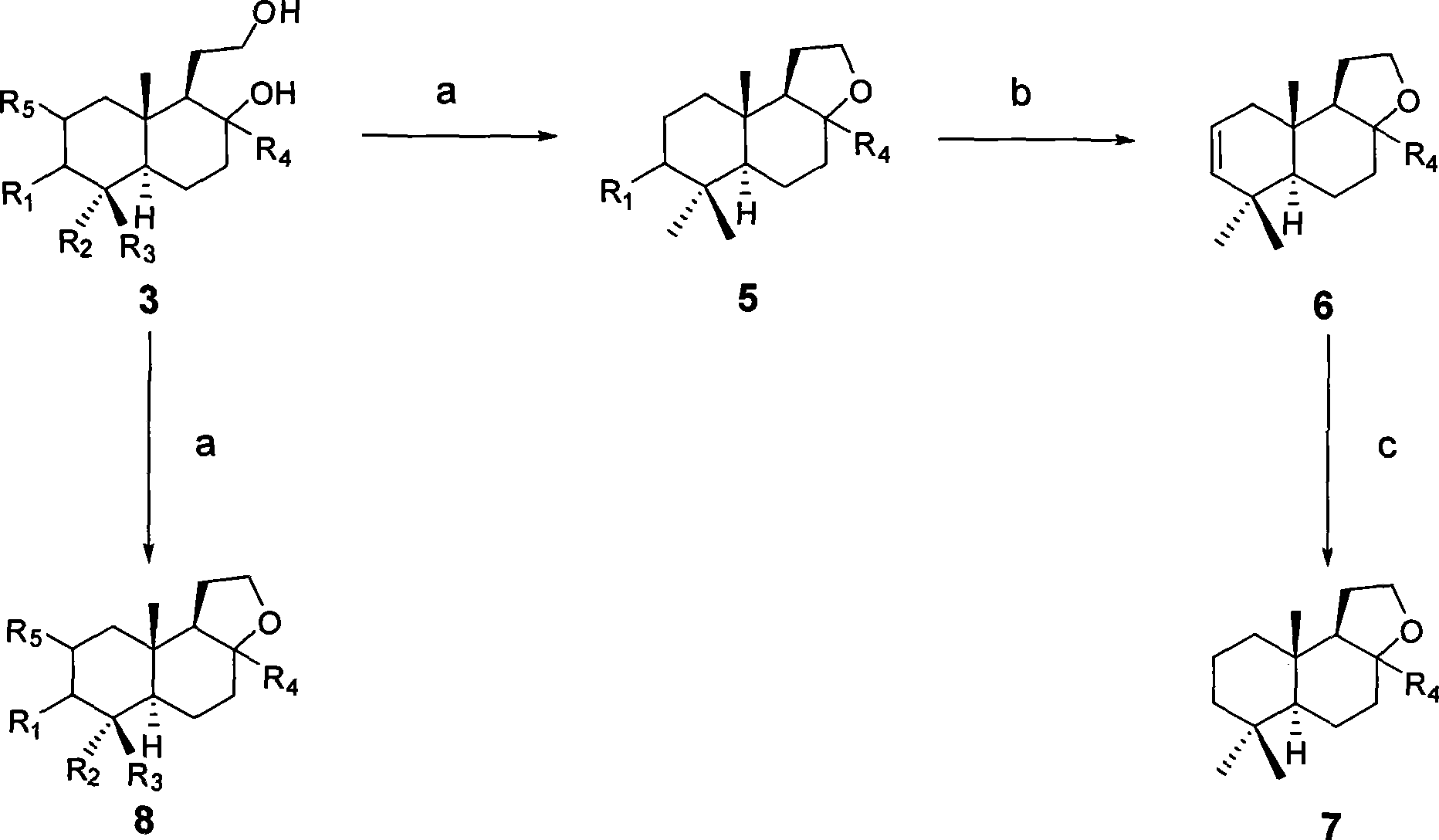
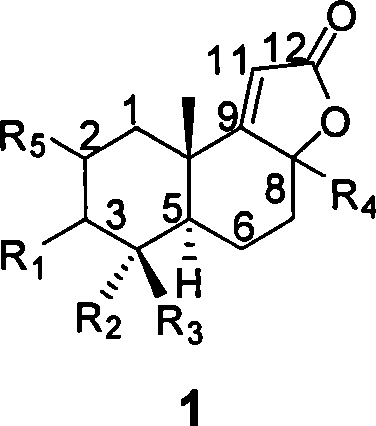
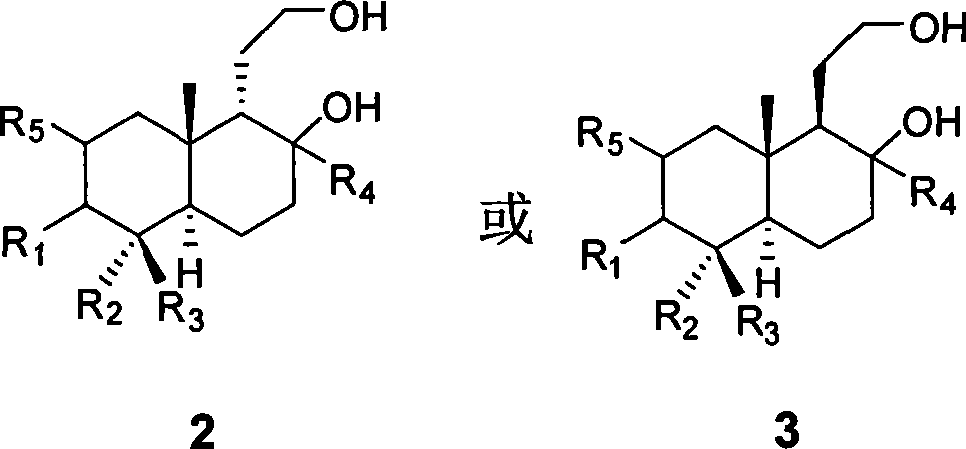
[0029] Example 1 With oleanolic acid AB (R 4 For β methyl) fragments as raw materials, synthetic compounds 2 and 3
[0030] 1) Preparation of Compound 2:
[0031]
[0032] LiAlH under the protection of argon 4 (49 mg, 1.3 mmol) was added into 5 mL of anhydrous THF dissolved in compound 1 (100 mg, 0.32 mmol) at room temperature, refluxed for 4 hours under the protection of argon, and then cooled to room temperature. Then 50 μL of water, 50 μL of 15% NaOH solution, and 150 μL of water were added sequentially to quench the reaction. After filtering and washing with THF three times, the combined filtrates were concentrated and then subjected to silica gel column chromatography (petroleum ether-acetone, 2:1) to obtain compound 2 (79 mg, 79%).
[0033] 2) Preparation of compound 4:
[0034]
[0035] Compound 1 (50 mg, 0.16 mmol) was added into the reaction flask, replaced with argon three times, then injected with 10 mL of anhydrous THF, and then injected with 135 μL of a ...
Embodiment 2
[0039] Example 2 With oleanolic acid AB (R 4 For α methyl) fragments as raw materials, synthetic compounds 2 and 3
[0040] Using the operation steps described in Example 1, the compound 2 and 3 obtained are as follows:
[0041]
Embodiment 3
[0042] Example 3 Using the AB fragment of maslinic acid as raw material to synthesize compounds 2 and 3
[0043] Using the operation steps described in Example 1, the compound 2 and 3 obtained are as follows:
[0044]
[0045] R 4 = α-methyl or β-methyl
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com