Preparation method of novel oleanane triterpenoid saponin derivative
A technology of oleanane-type and triterpene saponins, which is applied to the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of harsh reaction conditions, high cost, and many steps, and achieve mild reaction conditions, The effect of low price and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
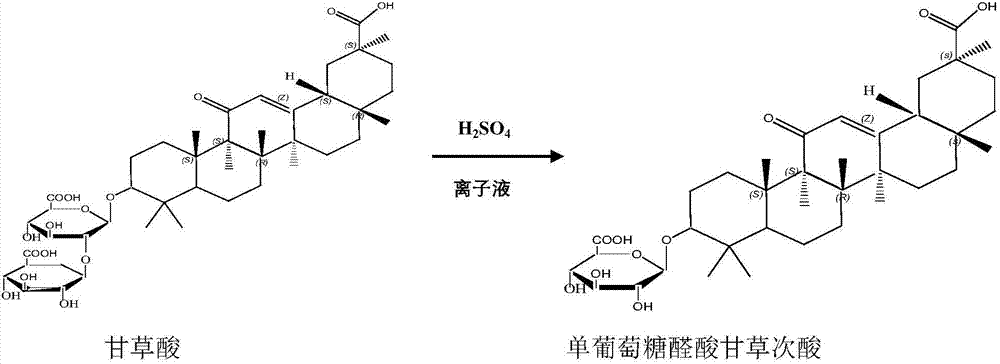
[0026] Weigh 10g of glycyrrhizic acid and place it in the reactor, then add 80g of [Emim] BF4 ionic liquid, stir to dissolve; add 3g of concentrated sulfuric acid, at a temperature of 30°C, the raw material is hydrolyzed by concentrated sulfuric acid under the ionic liquid system for 3 hours To obtain glycyrrhetinic acid monoglucuronide, adjust the pH of the reaction solution to 8 with ammonia water, after standing for stratification, separate the ionic liquid and the water phase in the reaction solution, and rotate the lower water phase to obtain a solid under reduced pressure. Dissolved in 80g of methanol, cooled and recrystallized to obtain 7.07g of pure glycyrrhetinic acid monoglucuronide, with a yield of 90%.
Embodiment 2
[0028] Weigh 10g of glycyrrhizic acid and place it in the reactor, then add 60g of [Emim] PF6 ionic liquid, stir to dissolve; add 4g of concentrated sulfuric acid, at a temperature of 40°C, the raw material is hydrolyzed by concentrated sulfuric acid under the ionic liquid system for 2 hours To obtain glycyrrhetinic acid monoglucuronide, adjust the pH of the reaction solution to 8 with ammonia water, after standing for stratification, separate the ionic liquid and the water phase in the reaction solution, and rotate the lower water phase to obtain a solid under reduced pressure. Dissolved in 80g of ethanol, cooled and recrystallized to obtain 7.30g of pure glycyrrhetinic acid monoglucuronide, with a yield of 93%.
Embodiment 3
[0030] Weigh 10g of glycyrrhizic acid and place it in the reactor, then add 100g of [Emim]ClO2 ionic liquid, stir to dissolve; add 4g of concentrated sulfuric acid, and at a temperature of 45°C, the raw material is hydrolyzed by concentrated sulfuric acid under the ionic liquid system for 2 hours To obtain glycyrrhetinic acid monoglucuronide, adjust the pH of the reaction solution to 9 with ammonia water, after standing for stratification, separate the ionic liquid and the water phase in the reaction solution, and rotate the lower water phase to obtain a solid under reduced pressure. Dissolved in 70g of glacial acetic acid, cooled and recrystallized to obtain 7.12g of pure glycyrrhetinic acid monoglucuronide, with a yield of 90.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com