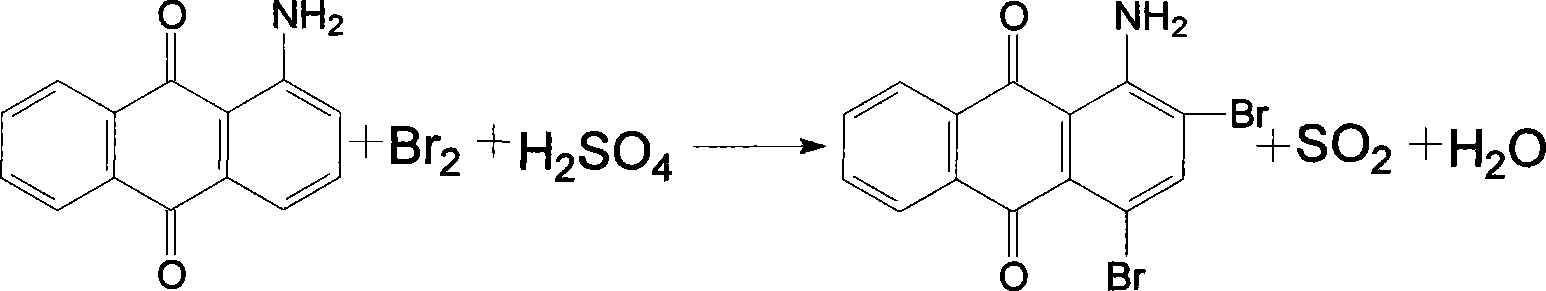
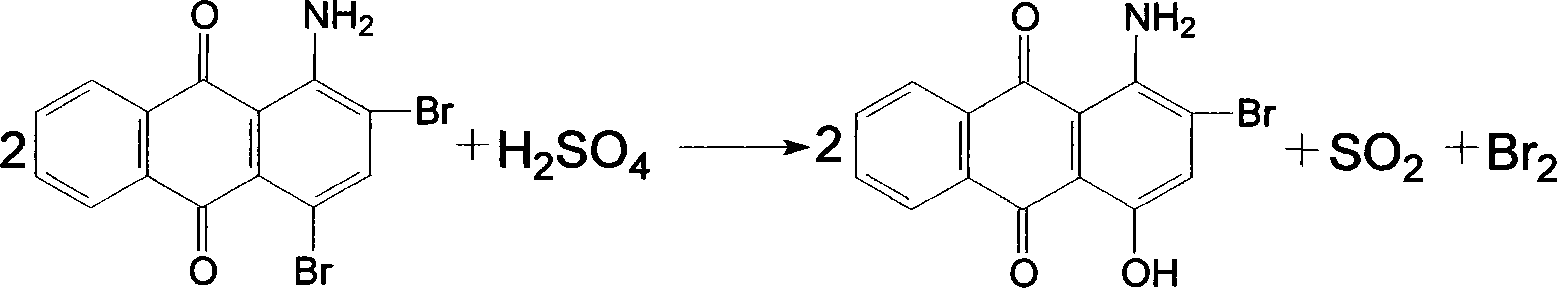
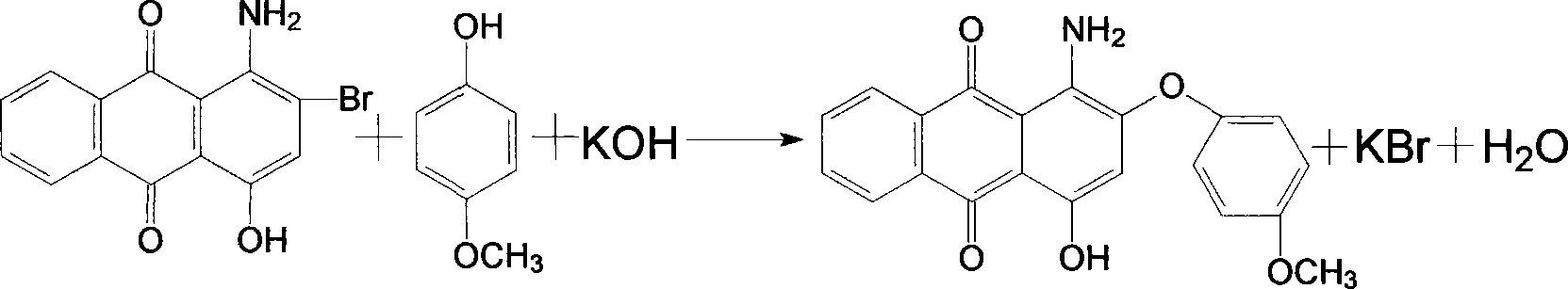
Dispersion red 146 condensation process improvement
A process improvement and disperse red technology, which is applied in the direction of amino-hydroxy anthraquinone dyes, etc., can solve the problems of long condensation reaction time, large product quality fluctuations, unfavorable condensation reaction, etc., and achieve shortened condensation reaction time, energy saving, and economic benefits obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add nitrobenzene 1500kg, chlorobenzene 500kg, 1-amino-2-bromo-4-hydroxyanthraquinone wet product filter cake to dry 450kg in the 3000L enamel kettle, heat up azeotropic dehydration, after the distillate separates the water layer, The solvent automatically flows back into the reaction kettle. After the distillate is anhydrous, add 180kg of p-hydroxyanisole, 100kg of acid-binding agent potassium hydroxide, and 50kg of catalyst tetrabutylammonium bromide. After the addition, the temperature is raised to 140~ 145°C, and keep it for 3 hours for condensation at this temperature. After the distillate is collected and separated during the heating and heat preservation process, the solvent is directly used for the next batch of condensation. After the end point is detected, the temperature is lowered to 75-80°C, and then the Transfer the material into a 5000L segregation tank, slowly add 1500kg of methanol dropwise at this temperature for about two hours, then cool down to below ...
Embodiment 2
[0025] Add 1500kg of nitrobenzene, 500kg of xylene, 1-amino-2-bromo-4-hydroxyanthraquinone wet product filter cake to 450kg in a 3000L enamel kettle, heat up and azeotropically dehydrate, and the distillate is separated from the water layer. The solvent automatically flows back into the reaction kettle. After the distillate is anhydrous, add 180kg of p-hydroxyanisole, 100kg of acid-binding agent potassium hydroxide, and 50kg of catalyst tetrabutylammonium bromide. After the addition, the temperature is raised to 140~ 145°C, and keep it for 3 hours for condensation at this temperature. After the distillate is collected and separated during the heating and heat preservation process, the solvent is directly used for the next batch of condensation. After the end point is detected, the temperature is lowered to 75-80°C, and then the Transfer the material into a 5000L segregation tank, slowly add 1500kg of methanol dropwise at this temperature for about two hours, then cool down to b...
Embodiment 3
[0027] Add 1800kg o-dichlorobenzene, 500kg chlorobenzene, 1-amino-2-bromo-4-hydroxyanthraquinone wet filter cake to 450kg in a 3000L enamel kettle, heat up azeotropic dehydration, and separate the distillate into the water layer , the solvent automatically flows back into the reactor, and after the distillate is anhydrous, 180kg of p-hydroxyanisole, 100kg of acid-binding agent potassium hydroxide, and 50kg of catalyst tetrabutylammonium bromide are added, and the temperature is raised to 140 within 3 hours after the addition. ~145°C, and keep it for 4 hours for condensation at this temperature. After the distillate is collected and stratified during the heating and holding process, the solvent is directly used for the next batch of condensation. After the end point is detected, the temperature is lowered to 75-80°C, and then Transfer the material into a 500L segregation kettle, slowly add 1500kg of methanol dropwise at this temperature for about two hours, then lower the temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com