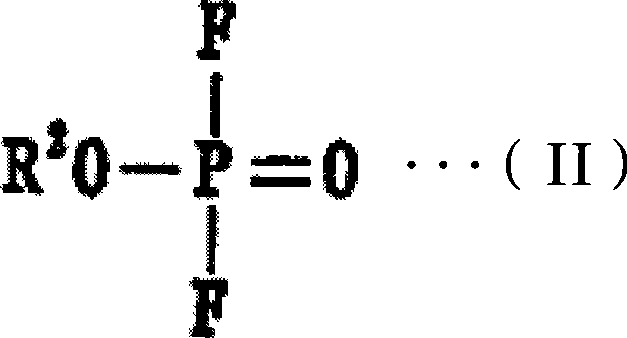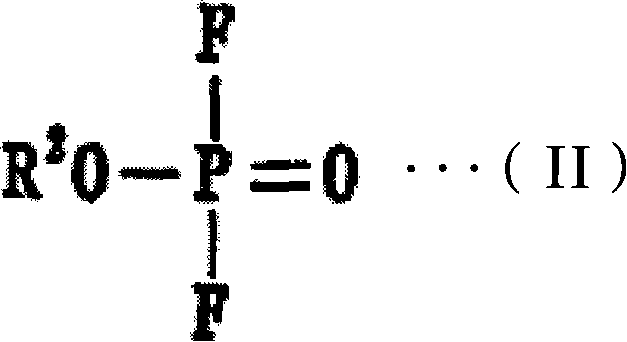Non-aqueous electrolyte for battery and non-aqueous electrolyte battery comprising the same
A non-aqueous electrolyte and battery technology, applied in non-aqueous electrolyte batteries, non-aqueous electrolytes, secondary batteries, etc., to achieve high safety and excellent battery characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] From 50% by volume of ethyl difluorophosphate, and 50% by volume of the above-mentioned general formula (I), n is 3, all R 1 Dissolve LiPF in an amount of 1 mol / L in a mixed solvent composed of cyclophosphazene compounds in which one is ethoxy and five are fluorine 6 , 3% by mass of 5-norbornene-2,3-dicarboxylic anhydride was added thereto to prepare a non-aqueous electrolytic solution. Next, the flame retardancy of the obtained non-aqueous electrolytic solution was evaluated by the following method, and the results shown in Table 1 were obtained.
[0061] (1) Evaluation of flame retardancy
[0062] Using the method stipulated in the UL94HB method of the UL (Underwriter Laboratories) standard, the burning length and burning time of the ignited flame were measured and evaluated in the air environment. Specifically, based on the UL test standard, the SiO 21.0 mL of the above electrolytic solution was infiltrated on the sheet, and a test piece was prepared and evaluated...
Embodiment 2
[0075] From 40% by volume of trifluoroethyl difluorophosphate, 20% by volume of the above-mentioned general formula (I), n is 3, all R 1 Among them, 2 are linked by ethylenedioxy, 4 are cyclic phosphazene compounds of fluorine, and 40% by volume of the above general formula (I), n is 4, all R 1 Dissolve LiPF in an amount of 1.2 mol / L in a mixed solvent composed of cyclophosphazene compounds in which one is ethoxy and seven are fluorine 6 , to which 3% by weight of 1,2,3,6-tetrahydrophthalic anhydride was added to prepare a non-aqueous electrolytic solution, and the flame retardancy of the obtained non-aqueous electrolytic solution was evaluated. In addition, a non-aqueous electrolyte secondary battery was produced in the same manner as in Example 1, and the cycle characteristics and safety under high voltage conditions were respectively evaluated. The results are shown in Table 1.
Embodiment 3
[0077] From 25% by volume of propyl difluorophosphate, 25% by volume of the above-mentioned general formula (I), n is 3, all R 1 In a mixed solvent composed of 3 methoxyl groups, 3 fluorine cyclic phosphazene compounds, 15% by volume ethylene carbonate, and 35% by volume dimethyl carbonate, dissolve LiPF in an amount of 1mol / L 6 , adding 2% by mass bicyclo[2.2.2]oct-5-ene-2,3-dicarboxylic acid anhydride to it to prepare a non-aqueous electrolyte, and evaluate the flame retardancy of the obtained non-aqueous electrolyte. In addition, a non-aqueous electrolyte secondary battery was produced in the same manner as in Example 1, and the cycle characteristics and safety under high voltage conditions were respectively evaluated. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com