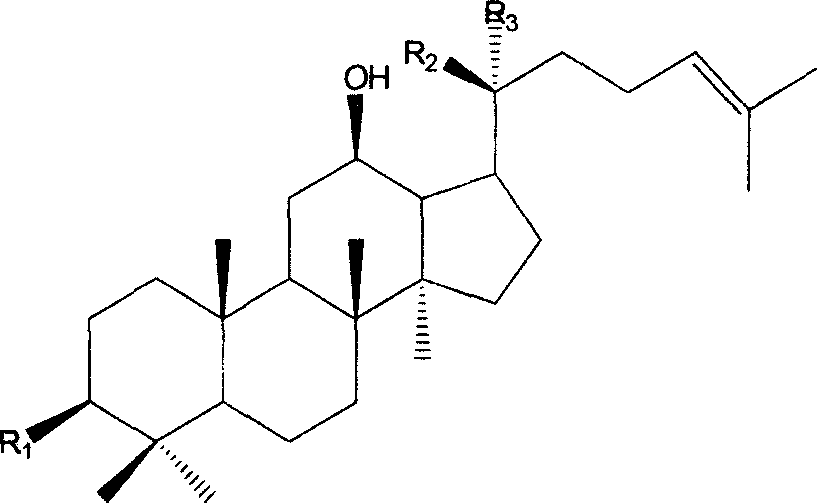
20(R)-ginsenoside composition and uses thereof
A technology of ginsenoside and composition, applied in the application field of medicine or health care product, can solve the problems of easy recurrence, lack of specificity of cytotoxicity, cell damage and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: capsule preparation
[0039] Weigh 20(R)-ginsenoside Rg 3 61.2 g, 20(R)-ginsenoside Rh 2 39.75 grams and 26.25 grams of ginsenoside III, add 867.8 grams of starch, mix well, sieve, use ethanol aqueous solution as binder to make a suitable soft material, granulate with 18 mesh sieve, dry in an oven at 60°C for 5 hours, 16 mesh Sieve the whole grain, add 5 grams of magnesium stearate, mix well, and make 1000 grains.
Embodiment 2
[0040] Embodiment 2: tablet preparation
[0041] Weigh 20(R)-ginsenoside Rg 3 75.3 g, 20(R)-ginsenoside Rh 2 29.25 grams and 24.75 grams of ginsenoside III, add 865.7 grams of starch, mix well, sieve, use ethanol aqueous solution as binder, make suitable soft material, granulate with 18 mesh sieve, dry in 60 ℃ oven for 5 hours, 16 mesh Sieve the whole grain, add 5 grams of magnesium stearate, mix well, and press into 1000 tablets.
Embodiment 3
[0042] Embodiment 3: preparation for injection
[0043] Weigh 20(R)-ginsenoside Rg 3 0.385 g, 20(R)-ginsenoside Rh 2 0.155 gram and 0.195 gram of ginsenoside III, add the propylene glycol of 20ml n-butanol and 30ml and make saponin dissolve completely, join the citric acid of 0.15 gram tetracaine hydrochloride, 0.02 gram in 40ml water for injection, make dissolving completely, and Add the above aqueous solution into the drug solution and mix well; adjust the pH value to 5.5±1.0 with borax and boric acid; add 0.1% activated carbon for needles and keep warm at 85°C for 30 minutes, filter with G3 vertical fused glass funnel, 0.22μm microporous filter membrane Filtrate; the filtrate is divided into 7ml vials, each with a volume of 2ml (the divided injections can be directly plugged and aluminum capped into injections; or freeze-dried by a freeze dryer to make freeze-dried powder injections).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com