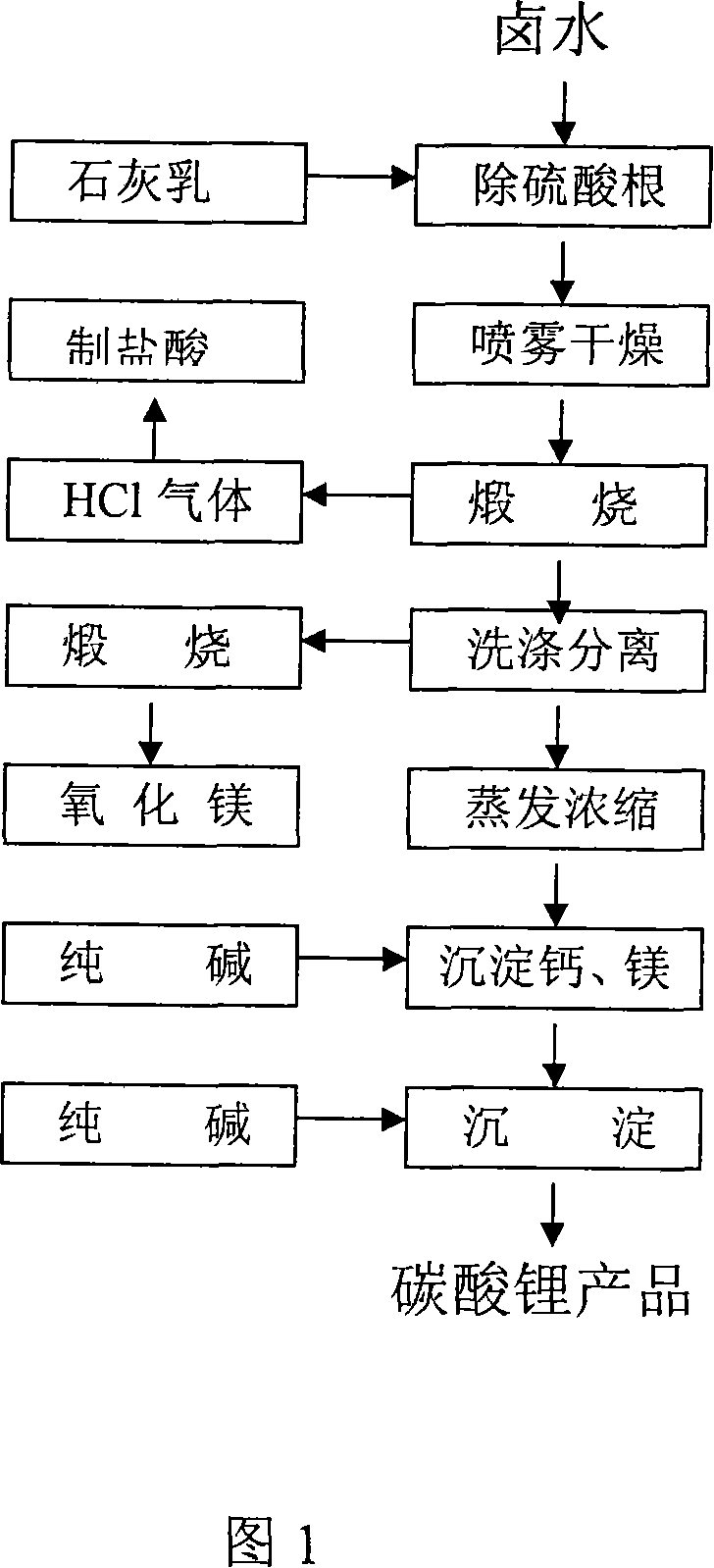
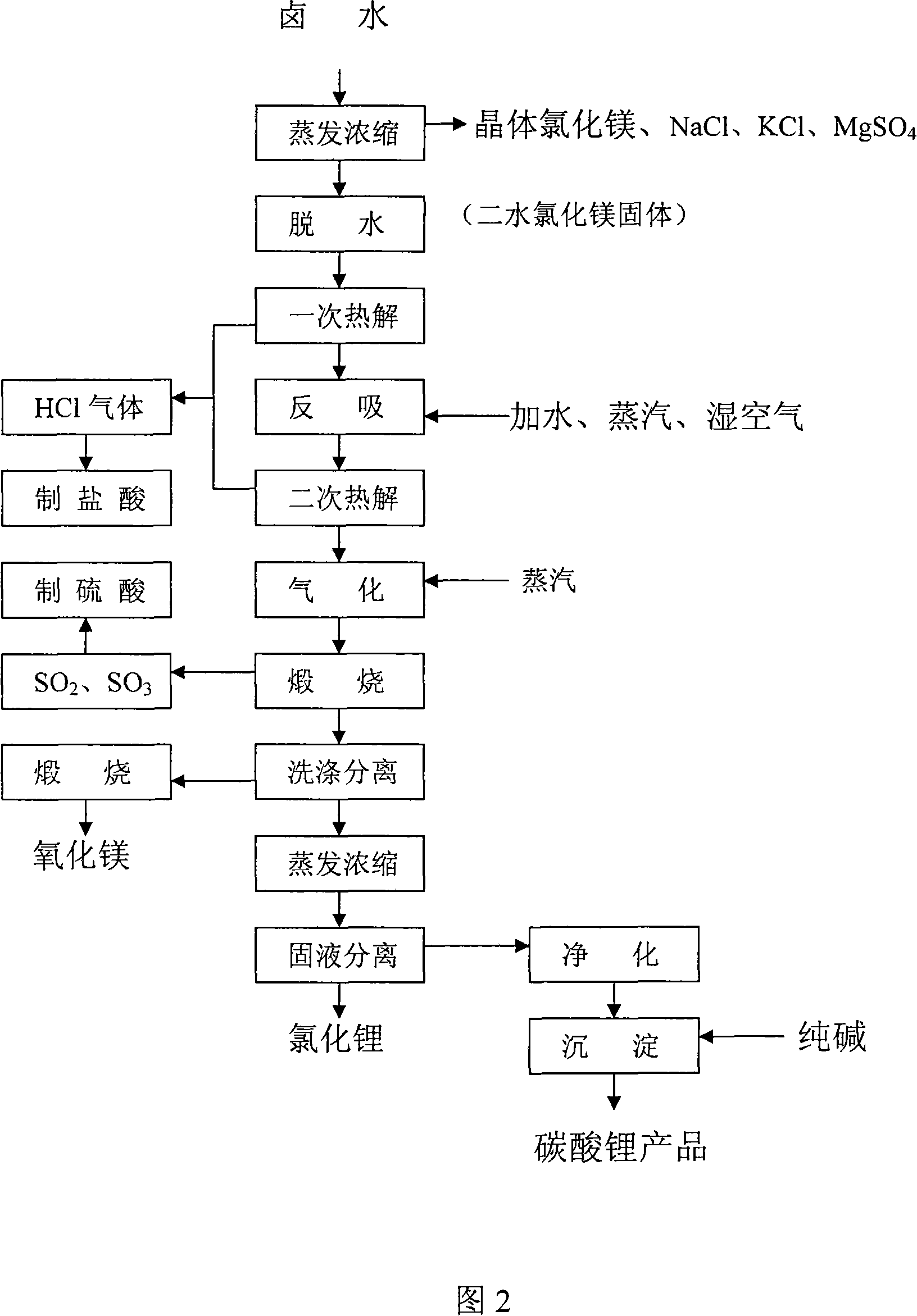
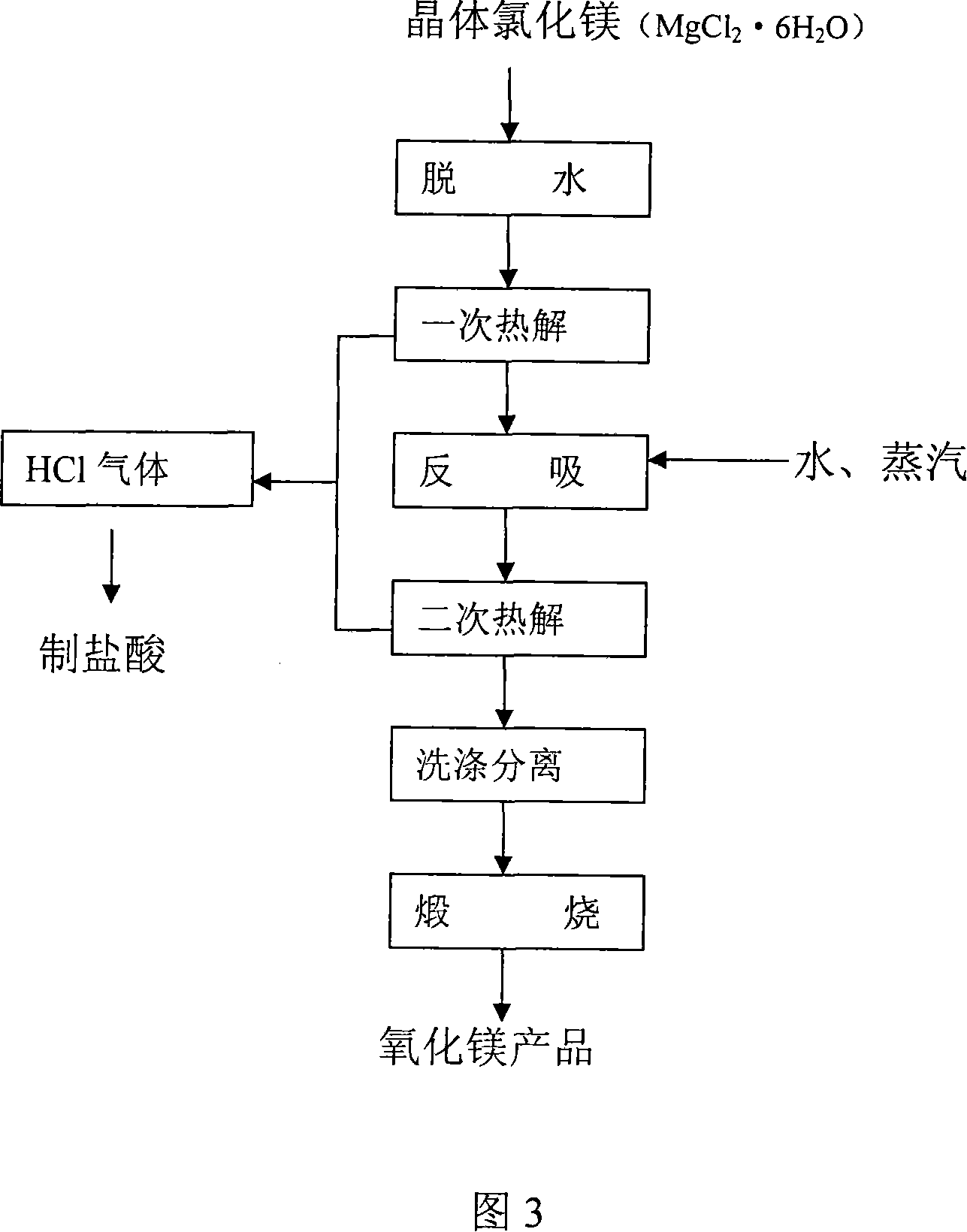
Process for producing high-purity magnesium oxide and lithium salt by using salt lake old brine
A technology of old brine and magnesium oxide in salt lakes, applied in magnesium oxide, carbonate preparations, alkali metal chlorides, etc., can solve the problems of large composition changes, incomplete separation, and low lithium salt acquisition rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Take 2000g of crystalline magnesium chloride obtained from the old brine through evaporative cooling, containing 45.2% magnesium chloride, put it in an oven, dehydrate at a constant temperature of 115°C to obtain magnesium chloride 4 hydrate, then raise the temperature to 160°C for dehydration at a constant temperature to obtain magnesium chloride dihydrate, enter One pyrolysis step, that is, continue to heat up to 230°C for dehydration at a constant temperature to obtain basic magnesium chloride, move the basic magnesium chloride to a muffle furnace for calcination at 600°C for 10 minutes, then take it out and cool it naturally to obtain a primary magnesium chloride with a MgO content of 57.5% and a mass of 502g. Thermally decompose the material, add water according to about 20% of the material weight, add 100g of water to the material while stirring, stir for 2 minutes, move it into the enamel plate to solidify for 4 hours, then grind it, pass through a 40-mesh sieve, a...
Embodiment 2
[0093] Take 10,000ml of old brine and evaporate it at room temperature to about 8,000ml, stir and add 30% industrial H 2 o 2 8ml, then add 40ml of 5% sodium hydroxide solution, cool naturally to room temperature, take 3000ml of the supernatant old brine, the old brine composition:
[0094] Mg Na K Li Cl SO 4
[0095] 117.93 0.75 1.13 6.2 360.73 23.25 (g / l)
[0096] Evaporate 3000ml of old brine at normal pressure, the evaporation temperature is 160°C, and naturally cool to room temperature. At this time, the evaporated material is in the form of lumps, crushed to pass through a 20-mesh sieve, put it in an oven for dehydration at 160°C, and then enter a pyrolysis step. That is, after heating up to 230°C for dehydration, move it to a muffle furnace, calcinate at 580°C for 30 minutes, take it out and cool it to room temperature naturally, add 150ml of water under stirring (reverse suction), solidify for 4 hours, grind, and pass through a 40-mesh sieve , and then put it into a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com