Method for preparing 3- bornylene
A technology of bornyl and bornylidene, which is applied in the field of preparation of 3-borneol-2-butanol, and can solve the problem of unstable aroma quality, incomplete or over-reaction, and rough finished product. Woody aroma and other problems, reaching the end of the reaction is easy to control, avoiding incomplete reaction, and good aroma effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
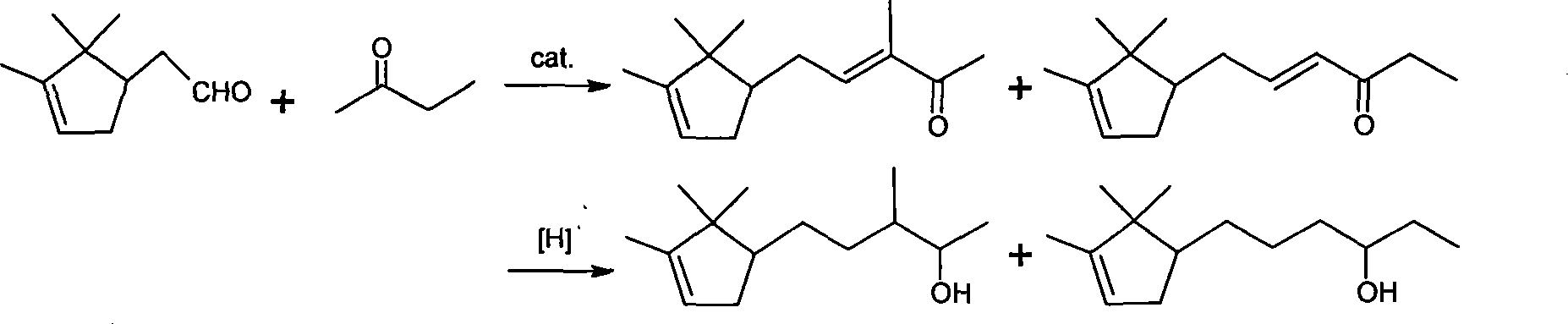
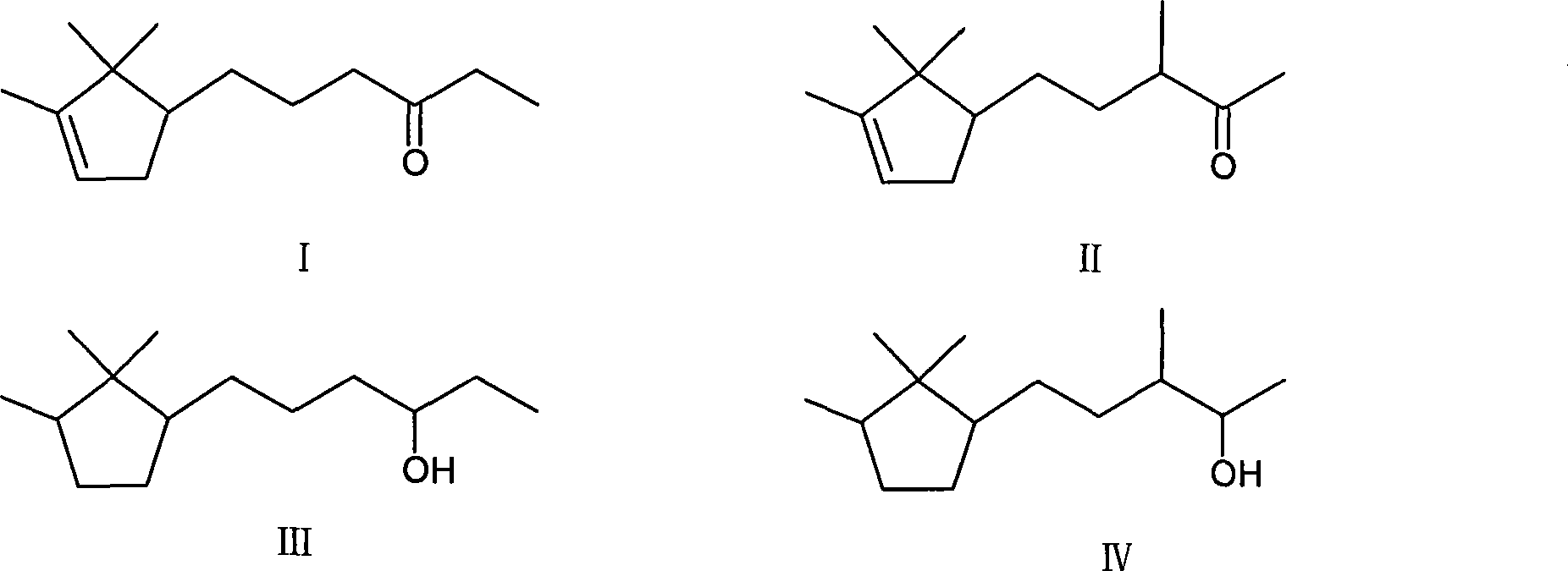
[0017] Embodiment 1: Preparation of 3-bornylidene-2-butanone
[0018] Add 350g (4.86mol) butanone, 800ml methyl alcohol, 350ml water and 30gKOH in the reaction kettle, add 200g (1.315mol) borneolenal under stirring, the reaction temperature is kept at room temperature, continue to react at room temperature for 8h after dropping, until The reaction is complete; the reactant is neutralized with acetic acid, and then washed with water until neutral. Remove solvent methanol and unreacted raw material butanone, distill under reduced pressure, collect fractions at 90-120°C / 2.5mmHg to obtain 243.8g of product with a yield of 90%.
Embodiment 2
[0019] Embodiment 2: the preparation of 3-borneolenyl-2-butanol crude product
[0020] Add 200g (0.97mol) 3-bornylidene-2-butanone, 160ml isopropanol, 0.06gKOH, 20g copper / chromium catalyst in the autoclave, the mixture is stirred and heated under 1~3MPa hydrogen pressure, and the temperature is maintained at Between 100°C and 200°C, react until gas chromatography shows that the content of raw materials or intermediates is 4.5%, stop the reaction, remove the solvent isopropanol in the reactant, distill under reduced pressure, and collect fractions at 103°C to 106°C / 133Pa, 185.4 g of product were obtained, a 91% yield.
Embodiment 3
[0021] Embodiment 3: the preparation of 3-borneolenyl-2-butanol crude product
[0022] Add 200g (0.97mol) 3-bornylidene-2-butanone in the autoclave, 180ml sec-butanol, 20g copper / chromium catalyst, 0.06gKOH, the mixture is stirred and heated under 1~3MPa hydrogen pressure, and the temperature is maintained at Between 110°C and 200°C, react until gas chromatography shows that the content of raw materials or intermediates is 4.3%, stop the reaction, remove the solvent sec-butanol in the reactant, distill under reduced pressure, collect fractions at 103°C to 106°C / 133Pa, 187 g of product was obtained, yield 91.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com