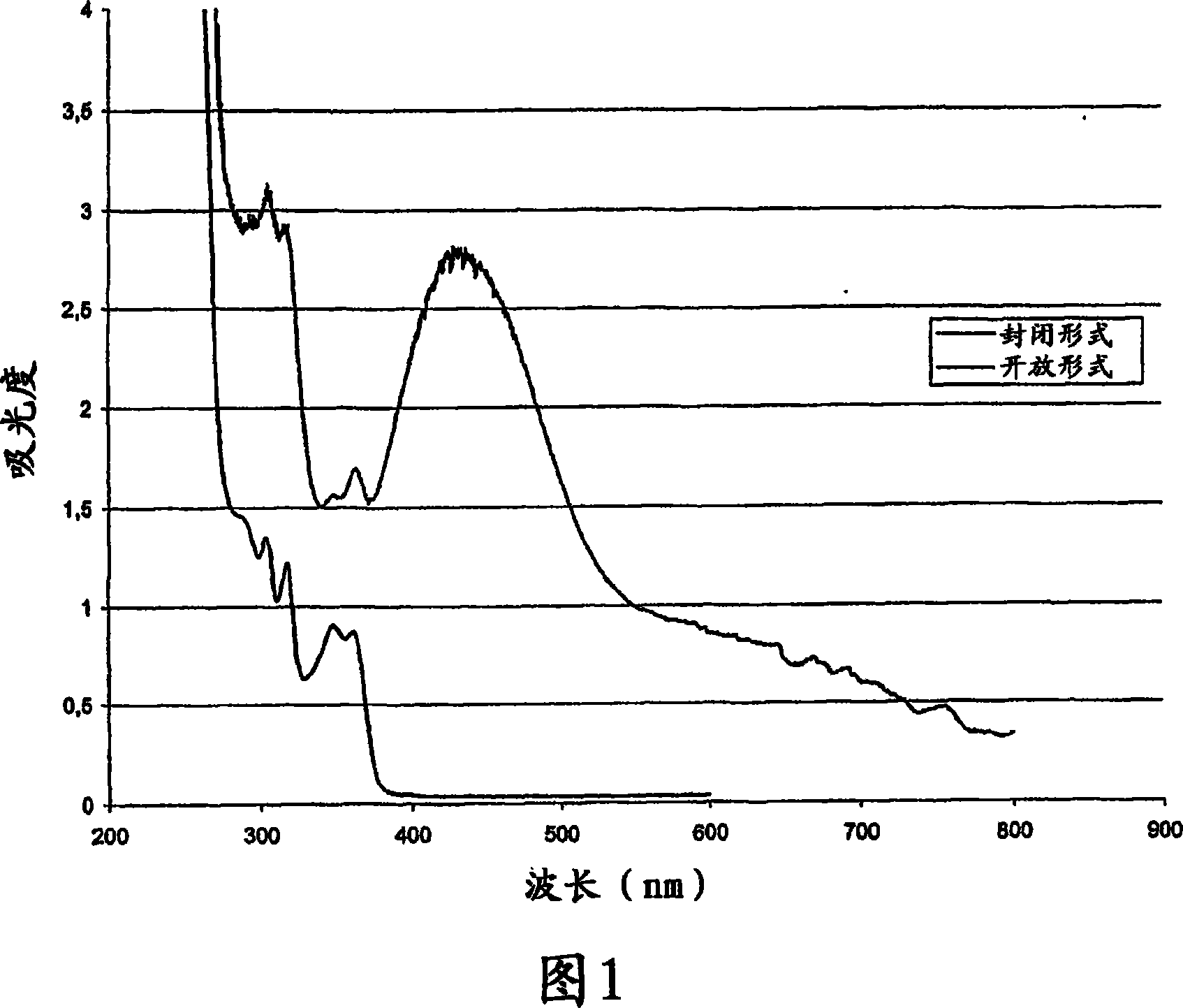
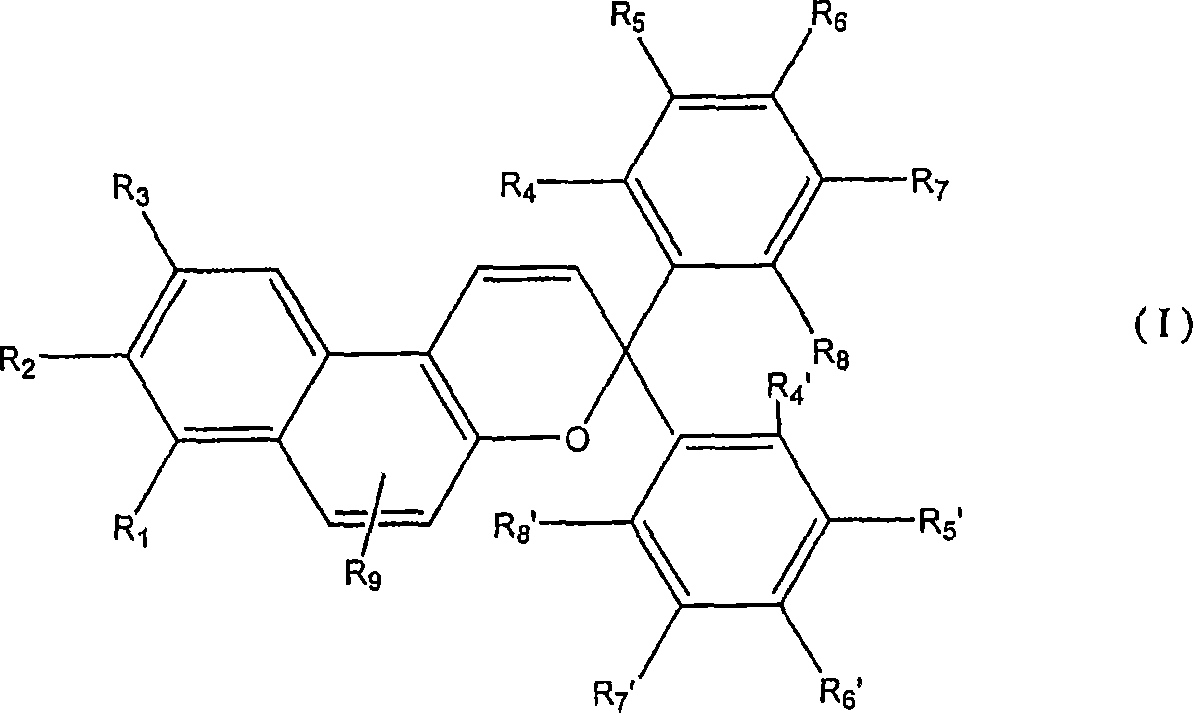
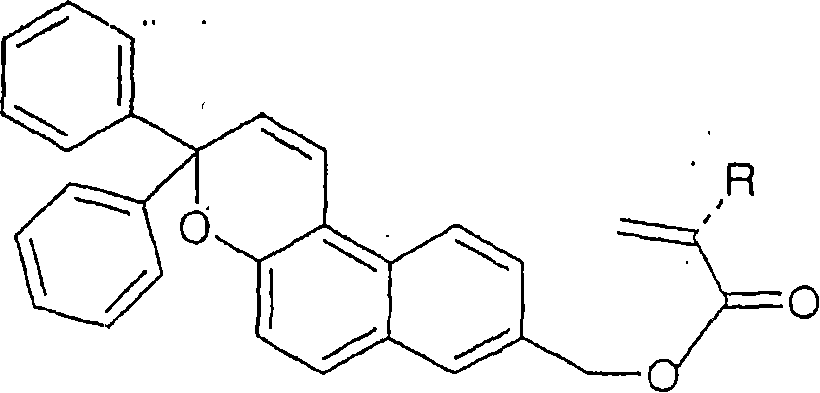
Photochromic intraocular lens
An intraocular lens and photochromic technology, applied in the direction of intraocular lens, optics, optical components, etc., can solve the problems of the wearer of the intraocular lens, such as obstacles and reduced vision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1: 8-Hydroxymethyl-3,3,diphenyl-3Hnaphtho[2,1]-pyran (compound (4)) Synthesis
[0093] This example is provided with reference to Synthesis Scheme n°1 above.
[0094] step 1 : Esterification of compound (1)
[0095] In a three-necked flask (250 ml) placed on a condenser, compound (1) (7 g) was dissolved in 80 ml of methanol. The esterification reaction was catalyzed by the addition of p-toluenesulfonic acid (APTS, 0.48 g), which was introduced under nitrogen. The reaction was brought to a temperature of 70°C and kept stirring for at least 12 hours.
[0096] At the end of this period, methanol was evaporated and the remaining organic phase was dissolved in ethyl acetate for liquid / liquid extraction (ethyl acetate / potassium carbonate saturated water) in order to purify the desired ester (compound (2)) .
[0097] The organic phase obtained by different washings was subsequently washed in MgSO 4 dried and then filtered. Evaporation of ethyl acetate allowe...
Embodiment 2
[0106] Example 2: Functionalization with Acryloyl Chloride: Compound (5)
[0107] In a three-necked flask (100ml) placed on the condenser, 1g of compound (4), 0.5ml of Et dissolved in 30ml of anhydrous THF were introduced 3 N (1.3 eq / (4)), addition of chloride was done dropwise in the presence of nitrogen at ambient temperature, no significant temperature increase was observed. The reaction was kept at ambient temperature with stirring for at least 12 hours.
[0108] At the end of this cycle, the solvent is evaporated. The residue was diluted in dichloromethane and extracted with successive washes of water saturated with potassium carbonate. Extract the organic phase using MgSO 4 Dry, filter on silica and solvent evaporated.
[0109] 3,3 Diphenyl-3Hnaphtho[2,1]-pyranacrylate was isolated in 60% mass yield.
Embodiment 3
[0110] Example 3: Functionalization with methacryloyl chloride
[0111] The synthesis was identical to that described in Example 2, except that methacryloyl chloride was substituted for acryloyl chloride.
[0112] 3,3Diphenyl-3Hnaphtho[2,1]-pyranylmethacrylate is thus obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com