Method for synthesizing 2-amido-6-chloropurine
A synthetic method, the technology of chloropurine, applied in the direction of organic chemistry, etc., can solve the problems of low product yield, side reactions, affecting the yield and purity of 2-amino-6-chloropurine, etc., and achieve a reasonable and high synthetic process. Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
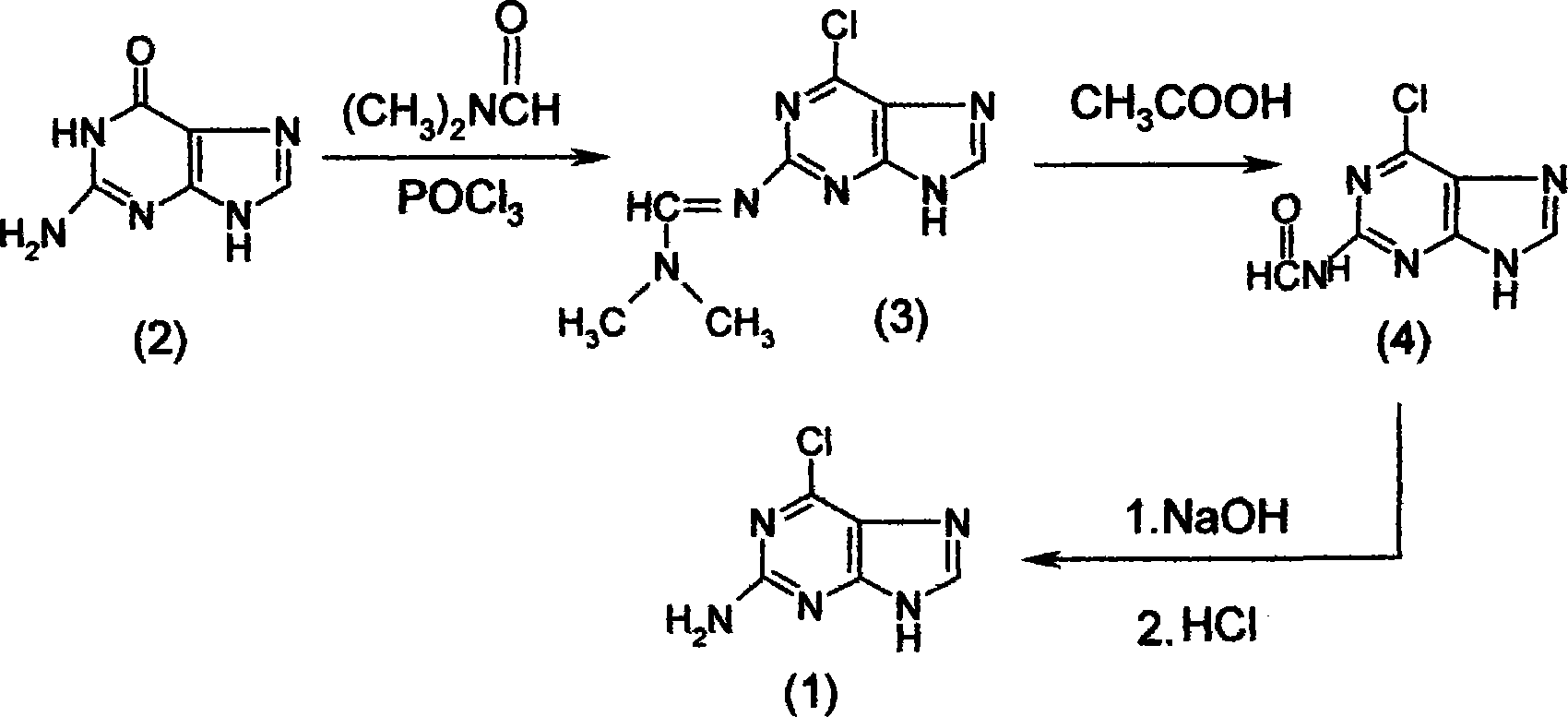
[0045] The synthesis of embodiment one 2-amino-6-chloropurine (1)
[0046] The synthetic method of 2-amino-6-chloropurine (1) comprises the following steps successively:
[0047] a) Preparation of 2-dimethylaminomethenimino-6-chloropurine (3)
[0048] First add 40mL of N,N-dimethylformamide to the dry reaction bottle, and add 35mL of POCl dropwise at 0℃~10℃ 3 , formulated as POCl 3 N,N-dimethylformamide solution, set aside; then in another dry reaction bottle, add 200mL of 1,2-dichloroethane and 20g of guanine (2), at 20℃~30℃ , dropwise add the prepared N,N-dimethylformamide solution of phosphorus oxychloride. After dripping, keep stirring for 1 hour, then heat up and reflux for 5 to 8 hours to complete the reaction, and cool to obtain 2-dimethylaminomethenimino-6-chloropurine (3). The intermediate product (3) does not need to be prepared from the reaction solution Separated from, the reaction solution containing the intermediate product (3) is for subsequent use;
[0049...
Embodiment 2
[0056] The synthesis of embodiment two 2-amino-6-chloropurine (1)
[0057] The synthetic method of 2-amino-6-chloropurine (1) comprises the following steps:
[0058] a) Preparation of 2-dimethylaminomethenimino-6-chloropurine (3)
[0059] First add 40mL of N,N-dimethylformamide to the dry reaction bottle, and add 35mL of POCl dropwise at 0℃~10℃ 3 , formulated as POCl 3 N,N-dimethylformamide solution, set aside; then in another dry reaction bottle, add 200mL of 1,2-dichloroethane and 20g of guanine (2), at 20℃~30℃ , dropwise add the prepared N,N-dimethylformamide solution of phosphorus oxychloride. After dripping, keep stirring for 1 hour, then heat up and reflux for 5 to 8 hours to complete the reaction, and cool to obtain 2-dimethylaminomethenimino-6-chloropurine (3). The intermediate product (3) does not need to be prepared from the reaction solution Separated from, the reaction solution containing the intermediate product (3) is for subsequent use;
[0060] b) Preparat...
Embodiment 3
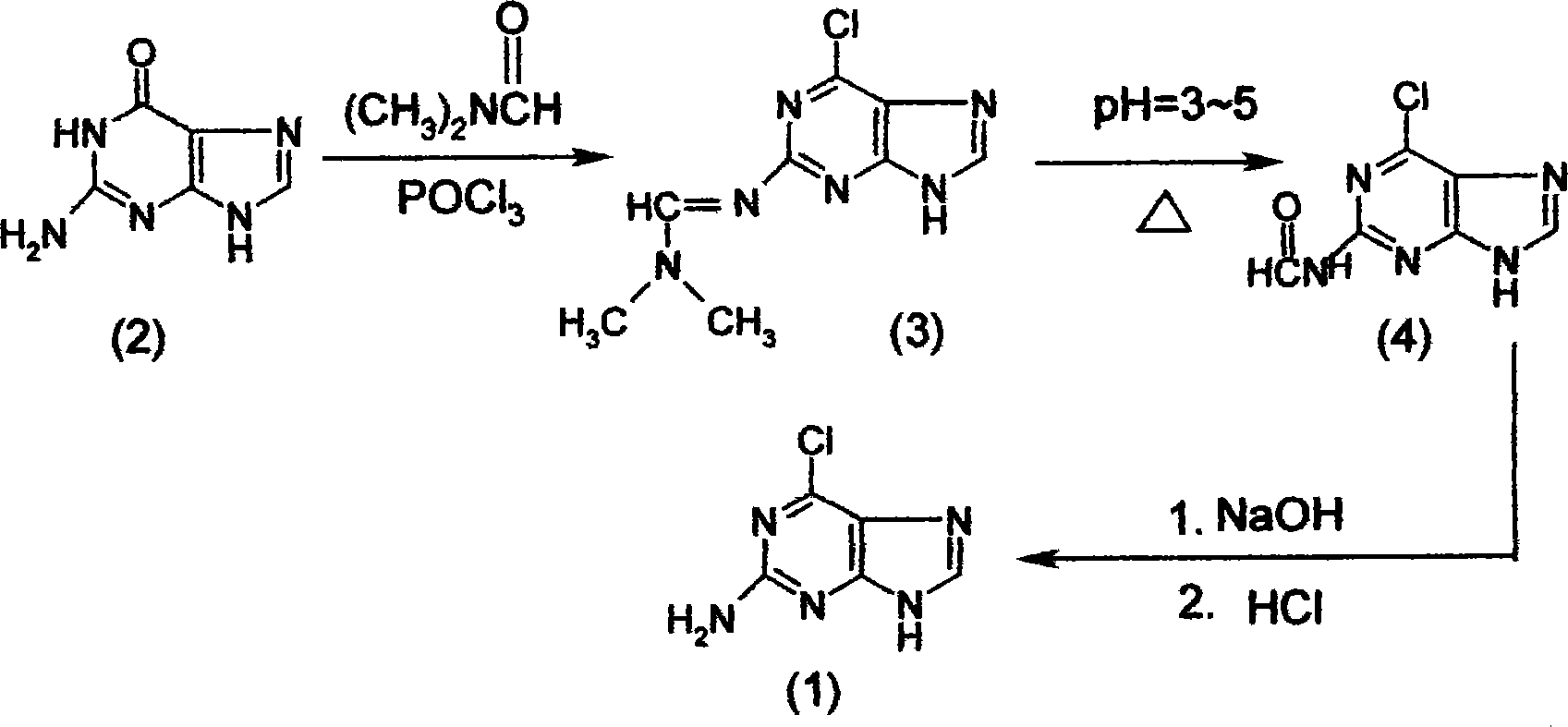
[0067] The synthesis of embodiment three 2-amino-6-chloropurine (1)
[0068] The synthetic method of 2-amino-6-chloropurine (1) comprises the following steps successively:
[0069] a) Preparation of 2-dimethylaminomethenimino-6-chloropurine (3)
[0070] First, add 40mL of N,N-dimethylformamide to the dry reaction bottle, and add 35mL of POCl dropwise at 0-10°C 3 , formulated as POCl 3 N,N-dimethylformamide solution, set aside; then in another dry reaction bottle, add 200mL of 1,2-dichloroethane and 20g of guanine (2), at 20℃~30℃ , dropwise add the prepared N,N-dimethylformamide solution of phosphorus oxychloride. After dripping, keep stirring for 1 hour, then heat up and reflux for 5 to 8 hours to complete the reaction, and cool to obtain 2-dimethylaminomethenimino-6-chloropurine (3). The intermediate product (3) does not need to be prepared from the reaction solution Separated from, the reaction solution containing the intermediate product (3) is for subsequent use;
[007...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com