Method for synthesizing 2-amido-6-chloropurine
A synthetic method, the technology of chloropurine, applied in the direction of organic chemistry, etc., can solve the problems of low product yield, difficulty in suction filtration, viscous material, etc., and achieve high yield and reasonable synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
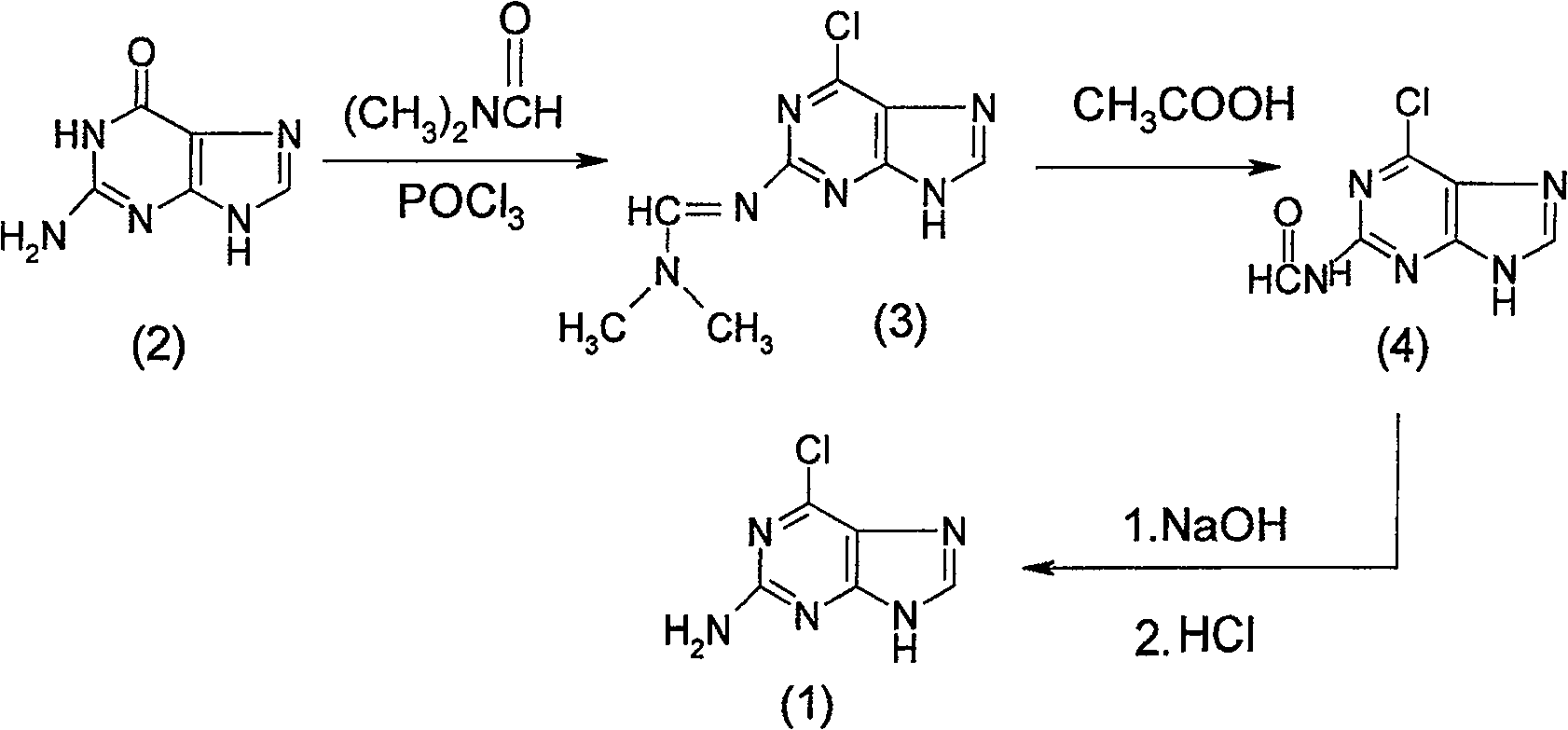
[0045] Example 1 Synthesis of 2-amino-6-chloropurine (1)
[0046] The synthetic method of 2-amino-6-chloropurine (1) comprises the following steps successively:
[0047] a) Preparation of 2-dimethylaminomethenimino-6-chloropurine (3)
[0048] First add 40mL of N,N-dimethylformamide to the dry reaction bottle, and add 35mL of POCl dropwise at 0℃~10℃ 3 , formulated as POCl 3 N,N-dimethylformamide solution, set aside; then in another dry reaction bottle, add 200mL of 1,2-dichloroethane and 20g of guanine (2), at 20℃~30℃ , dropwise add the prepared N,N-dimethylformamide solution of phosphorus oxychloride. After dripping, keep stirring for 1 hour, then heat up and reflux for 5 to 8 hours to complete the reaction, and cool to obtain 2-dimethylaminomethenimino-6-chloropurine (3). The intermediate product (3) does not need to be obtained from the reaction solution Separated from, the reaction solution containing the intermediate product (3) is for subsequent use;
[0049] b) Prep...
Embodiment 2
[0056] The synthesis of embodiment two 2-amino-6-chloropurine (1)
[0057] The synthetic method of 2-amino-6-chloropurine (1) comprises the following steps:
[0058] a) Preparation of 2-dimethylaminomethenimino-6-chloropurine (3)
[0059] First add 40mL of N,N-dimethylformamide to the dry reaction bottle, and add 35mL of POCl dropwise at 0℃~10℃ 3 , formulated as POCl 3 N,N-dimethylformamide solution, set aside; then in another dry reaction bottle, add 200mL of 1,2-dichloroethane and 20g of guanine (2), at 20℃~30℃ , dropwise add the prepared N,N-dimethylformamide solution of phosphorus oxychloride. After dripping, keep stirring for 1 hour, then heat up and reflux for 5 to 8 hours to complete the reaction, and cool to obtain 2-dimethylaminomethenimino-6-chloropurine (3). The intermediate product (3) does not need to be prepared from the reaction solution Separated from, the reaction solution containing the intermediate product (3) is for subsequent use;
[0060] b) Preparat...
Embodiment 3
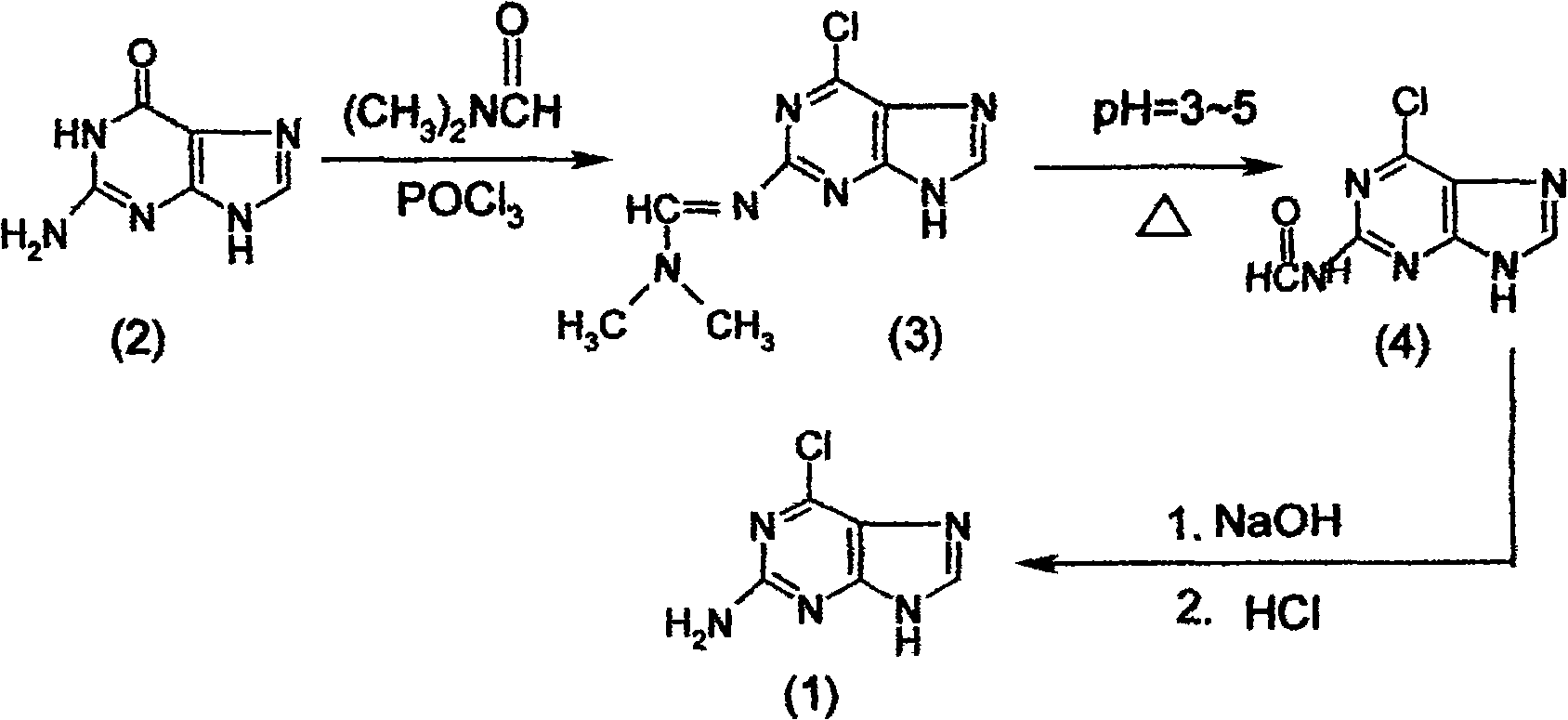
[0067] Example 3 Synthesis of 2-amino-6-chloropurine (1)
[0068] The synthetic method of 2-amino-6-chloropurine (1) comprises the following steps successively:
[0069] a) Preparation of 2-dimethylaminomethenimino-6-chloropurine (3)
[0070] First, add 40mL of N,N-dimethylformamide to the dry reaction bottle, and add 35mL of POCl dropwise at 0-10°C 3 , formulated as POCl 3 N,N-dimethylformamide solution, set aside; then in another dry reaction bottle, add 200mL of 1,2-dichloroethane and 20g of guanine (2), at 20℃~30℃ , dropwise add the prepared N,N-dimethylformamide solution of phosphorus oxychloride. After dripping, keep stirring for 1 hour, then heat up and reflux for 5 to 8 hours to complete the reaction, and cool to obtain 2-dimethylaminomethenimino-6-chloropurine (3). The intermediate product (3) does not need to be prepared from the reaction solution Separated from, the reaction solution containing the intermediate product (3) is for subsequent use;
[0071] b) Pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com