Fused protein restructuring target lethal leukemia cells and preparation method and use thereof
A leukemic cell and fusion protein technology, applied in the direction of drug combination, peptide/protein components, recombinant DNA technology, etc., to achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation method of plasmid pGEX-4T-1-PE38KDEL
[0031] 1. Amplification of the PEA gene
[0032] Chromosomal DNA of Pseudomonas aeruginosa PA103 was extracted according to conventional methods. A pair of primers located on both sides of the PEA structural gene were designed according to the sequence published by Gray et al.
[0033] 5' Primer: 5'-GAT CAG CCT CAT CCT TCA C-3',
[0034] 3' primer: 5'-GCT CGC GGC AGT TAC TT-3'.
[0035] Reaction system: 5 μl DNA template (0.5 μg), 5 μl 10×PCR amplification buffer, 2 μl (100 ng) each of 5’ primer and 3’ primer, 1 μl of 10 mmol / L dNTP mixture, 5 μl of 50% glycerol, 2.5 μl of DMSO, Bacteria water 27μl, TaqDNA polymerase 0.5μl (2.5u), total volume 50μl. Reaction conditions: Denaturation at 94°C for 4 wins, followed by cycling, denaturation at 94°C for 1 min, annealing at 58°C for 1 min, extension at 72°C for 3 min, and a total of 30 cycles. Final extension at 72°C for 30 min.
[0036] 2. PE40 gene fragment...
Embodiment 2
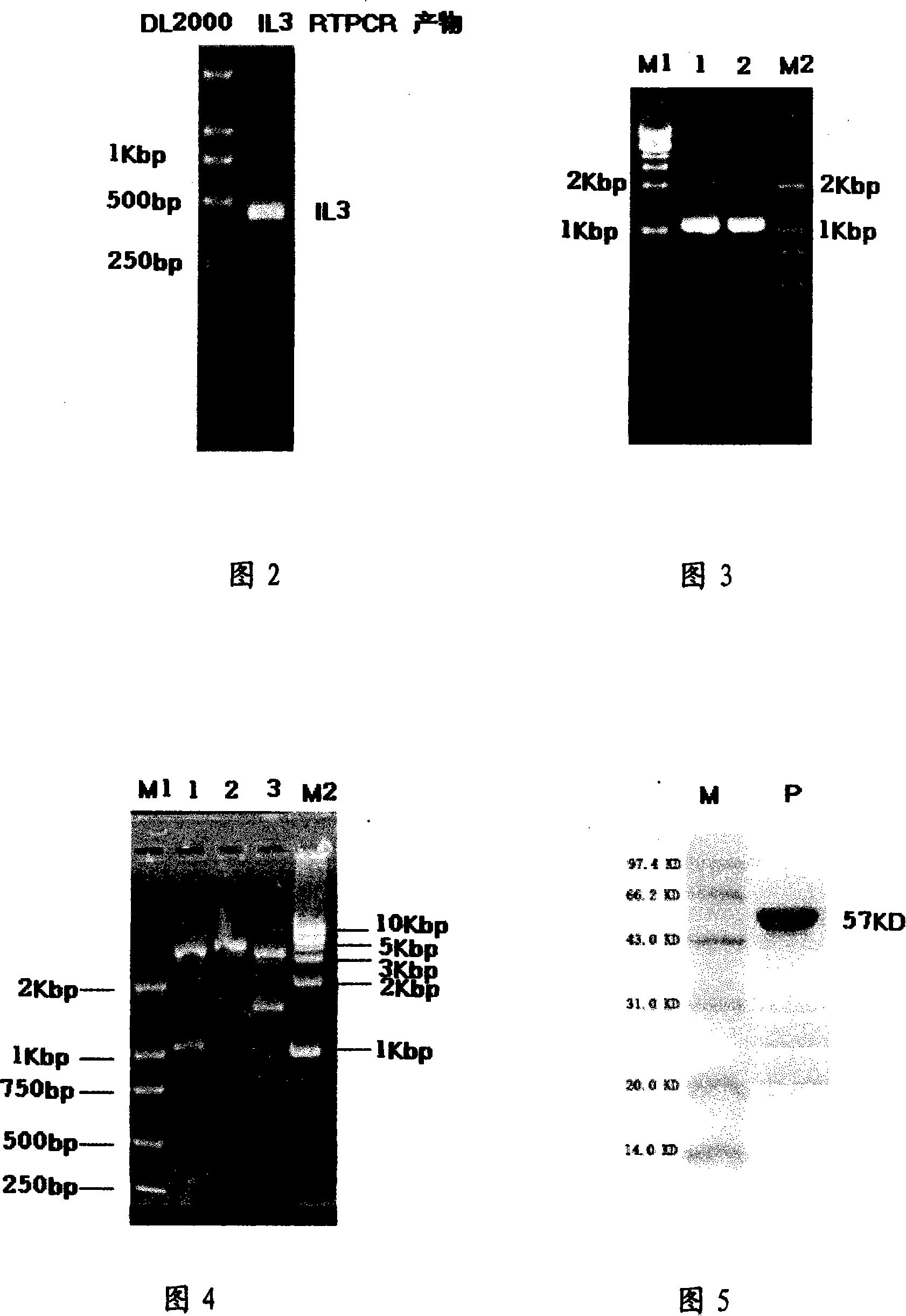
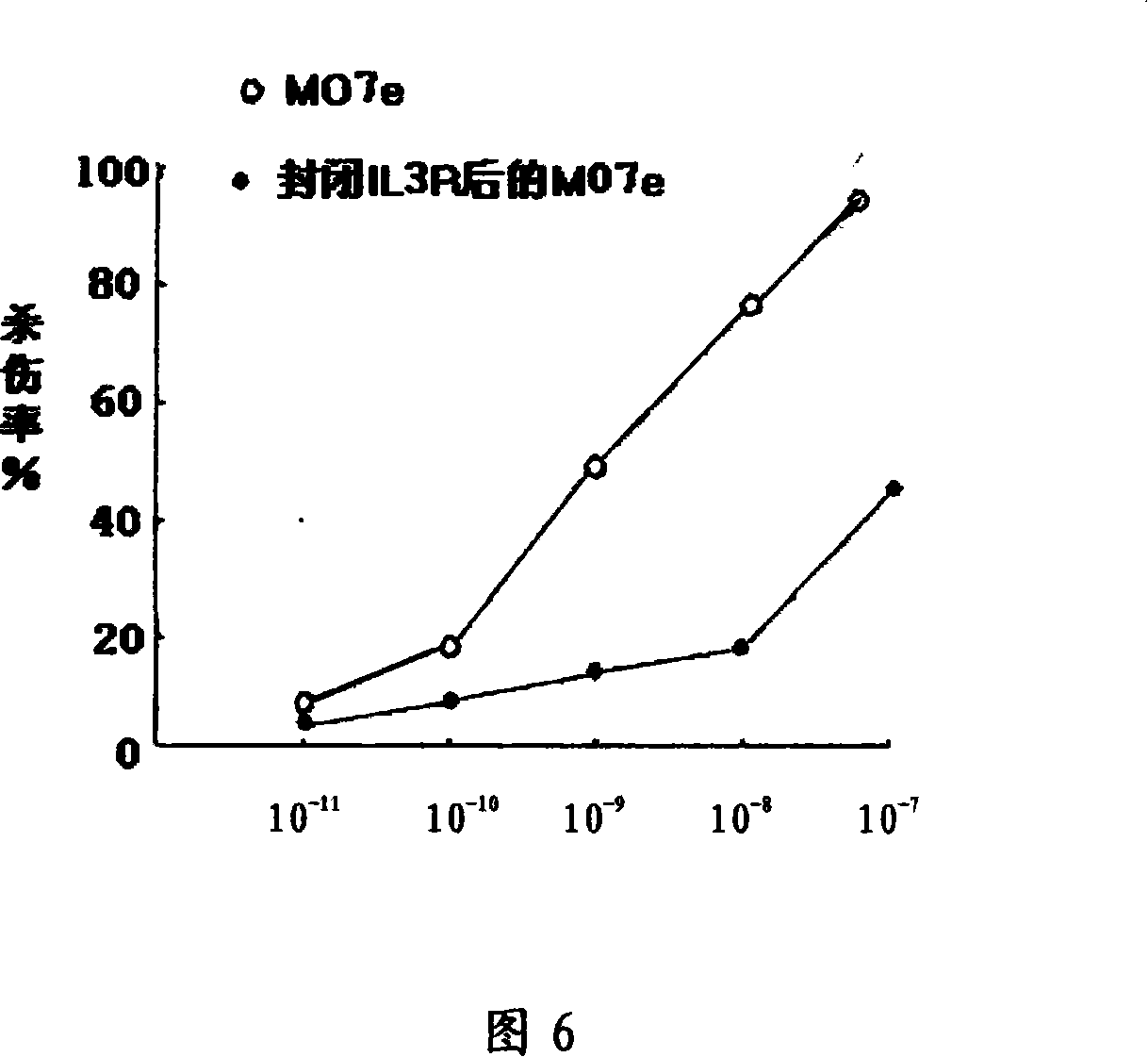
[0052] Example 2: Expression and Activity Identification of Recombinant Protein IL3-PE38KDEL
[0053] 1. Materials and methods
[0054] 1.1 Reagents
[0055] Various restriction endonucleases, TaqDNA polymerase, PrimeSTARTM HS DNA Polymerase, DL2000Marker, 500bpMarker, protein marker, T4 ligase, plasmid mini-pumping kit, and gel recovery reagents were all purchased from Takara Company, and the primers were synthesized by Shanghai Ying Jun company completed.
[0056] IPTG, Coomassie Brilliant Blue R250 were purchased from Takara Company.
[0057] Nickel ion affinity chromatography column (Ni2+-NTA) was purchased from Novagen.
[0058] RPMI1640 was purchased from GIBCO Company.
[0059] Calf serum was purchased from Hangzhou Sijiqing Biomaterials Co., Ltd.
[0060] MTT is a product of Amresco.
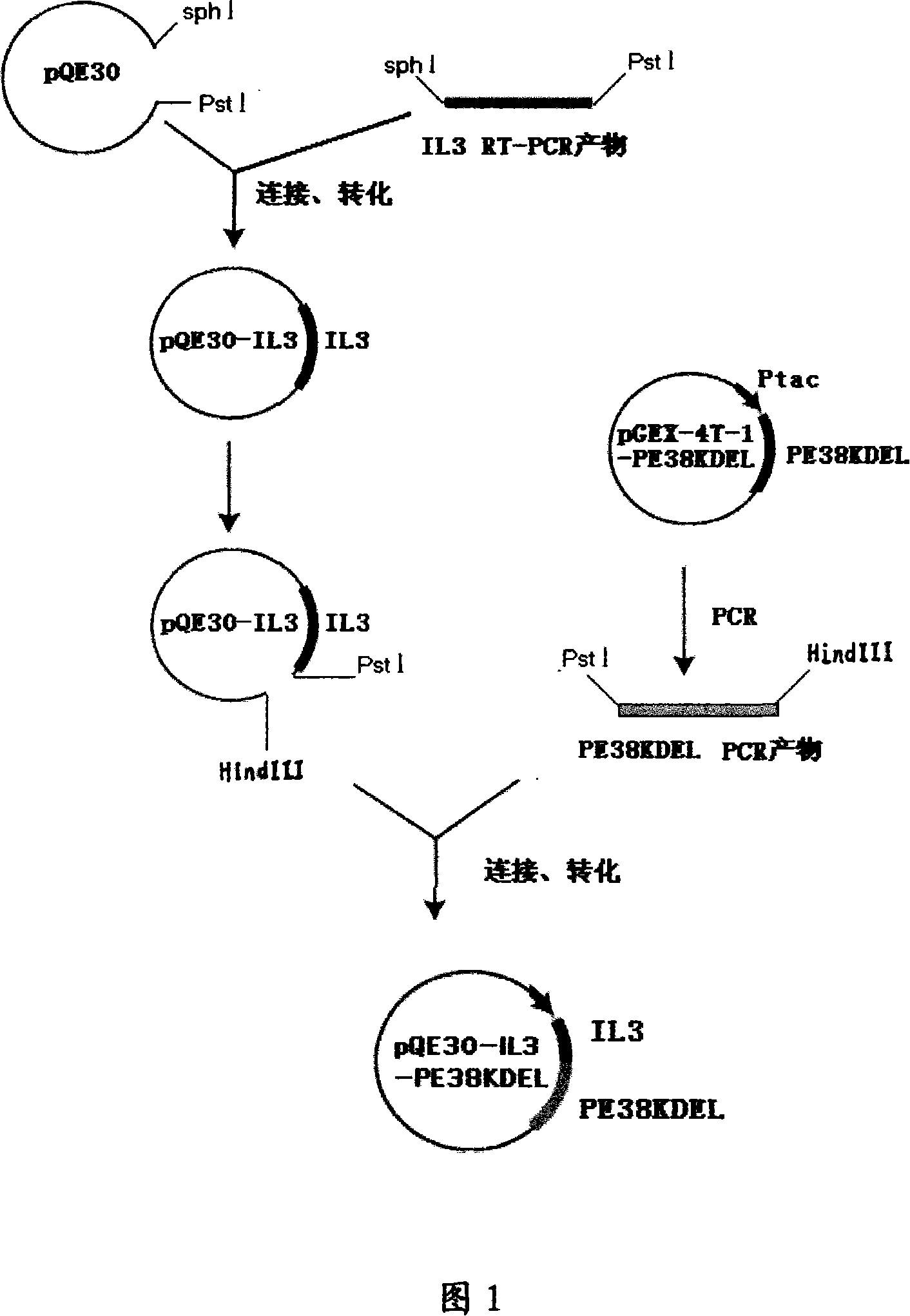
[0061] 1.2 Construction of fusion gene PQE30-IL3-Linker-PE38KDEL
[0062] 1.2.1 Construction of recombinant plasmid pQE30-Linker
[0063]In order to ensure that the protein exp...
Embodiment 3
[0094] Example 3: Expression and Activity Identification of Recombinant Protein IL3-Linker-PE38KDEL
[0095] 1. Materials and methods
[0096] 1.1 Reagents
[0097] Various restriction enzymes, TaqDNA polymerase, PrimeSTAR TM HS DNA Polymerase enzyme, DL2000Marker, 500bpMarker, protein marker, T4 ligase, plasmid mini-pump kit, and gel recovery reagent were all purchased from Takara Company.
[0098] The synthesis of primers was completed by Shanghai Yingjun Company.
[0099] IPTG, Coomassie Brilliant Blue R250 were purchased from Takara Company.
[0100] Nickel ion affinity chromatography column (Ni 2+ -NTA) was purchased from Novagen.
[0101] RPMI1640 was purchased from GIBCO Company.
[0102] Calf serum was purchased from Hangzhou Sijiqing Biomaterials Co., Ltd.
[0103] MTT is a product of Amresco.
[0104] 1.2 Synthetic primers:
[0105] Primerl (upstream) 5'-TCGC GCATGC GCTCCCATGACCCAG-3' (the underline is the sphI restriction site),
[0106] Primer2 (down...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com