Preparation method and application of fusion protein and vaccine composition containing same
A vaccine composition and fusion protein technology, applied in the field of veterinary biological products, can solve the problems of immune failure, long research and development cycle, and slow antibody production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The synthesis of embodiment 1NLNK gene
[0059] In order to make the fusion protein have a good spatial structure and free extension, and improve the pharmacological and biological activities, a hydrophobic flexible peptide (GGGGSGGGGSGGGGS) composed of glutamic acid and serine was designed as a Linker to connect between Nsp9 and Nsp10.
[0060] According to the Nsp9 protein and Nsp10 protein in the highly pathogenic porcine reproductive and respiratory syndrome virus JXA1 strain (accession number: FJ548854.1) reported in NCBI (http: / / www.ncbi.nlm.nih.gov) The gene sequence, as well as the gene sequence of Linker (GGGGSGGGGSGGGGS) and the carboxy-terminal part (KKDELRVELKDEL), were synthesized by Shanghai Bioengineering Co., Ltd. by artificial synthesis, and the synthesized gene was named Nsp9-Linker-Nsp10-KDEL (abbreviated as NLNK ), the synthesized gene fragment has a full length of 243bp, and the sequencing result is shown in SEQ ID No.8 of the sequence table.
Embodiment 2
[0061] Example 2 Preparation, Identification and Purification of Fusion Protein PEA-NLNK
[0062] 2.1 Synthesis and amplification of PEA gene
[0063] According to the gene sequence of Pseudomonas aeruginosa exotoxin type A (Pseudomonas aeruginosa exotoxin type A, accession number: K01397.1) domain I and II reported in NCBI (http: / / www.ncbi.nlm.nih.gov) ( See SEQ ID NO.9), and use PCR to amplify the corresponding target gene. Wherein, the PEA gene primer design is as follows:
[0064] Upstream primer F1: 5'-CGGGATCCGCCGAGGAAGCCTTCGAC-3',
[0065] Downstream primer R1: 5'-CAACTTCCTCTTTGCCGTCGCCGAGGAACT-3',
[0066] PCR amplification program: 95°C pre-denaturation for 5 min, 94°C denaturation for 45 s, 52°C renaturation for 30 s, 72°C extension for 60 s, 35 cycles, and finally 72°C extension for 10 min.
[0067] The obtained PCR amplification product was electrophoresed with 1% agarose gel, and the detection result showed that the corresponding target band appeared at about ...
Embodiment 3
[0085] Embodiment 3 Preparation of highly pathogenic porcine reproductive and respiratory syndrome vaccine composition
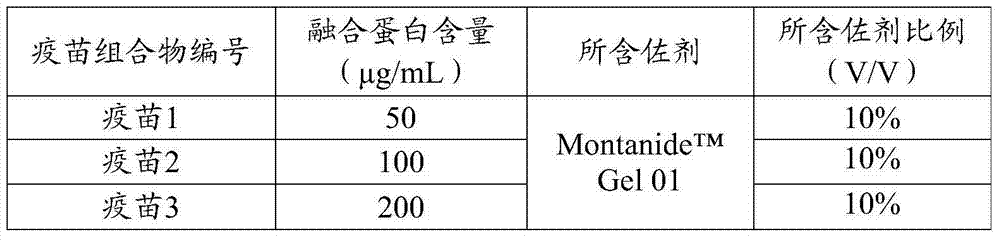
[0086] Dilute the fusion protein PEA-NLNK prepared in Example 2 with a pH7.2 PBS solution, prepare the diluted fusion protein solution and Gel adjuvant as shown in Table 1, stir at a speed of 500-800r / min for 10-15min, Add 1% thimerosal solution by volume before terminating the stirring so that the final concentration does not exceed 1 / 10,000, shake and mix well, and the highly pathogenic porcine reproductive and respiratory syndrome vaccine composition is obtained. Store it at 2-8°C after aliquoting.
[0087] Table 1 Components and ratios contained in the highly pathogenic porcine reproductive and respiratory syndrome vaccine composition
[0088]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com