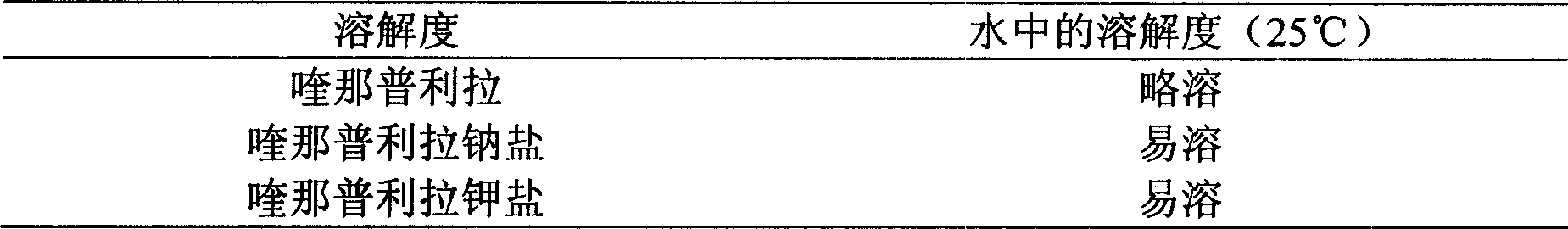
Metal salt of quinaprilat
A technology of quinapril and metal salt, applied in the field of medicine, can solve the problems of poor water solubility and poor stability of quinapril
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
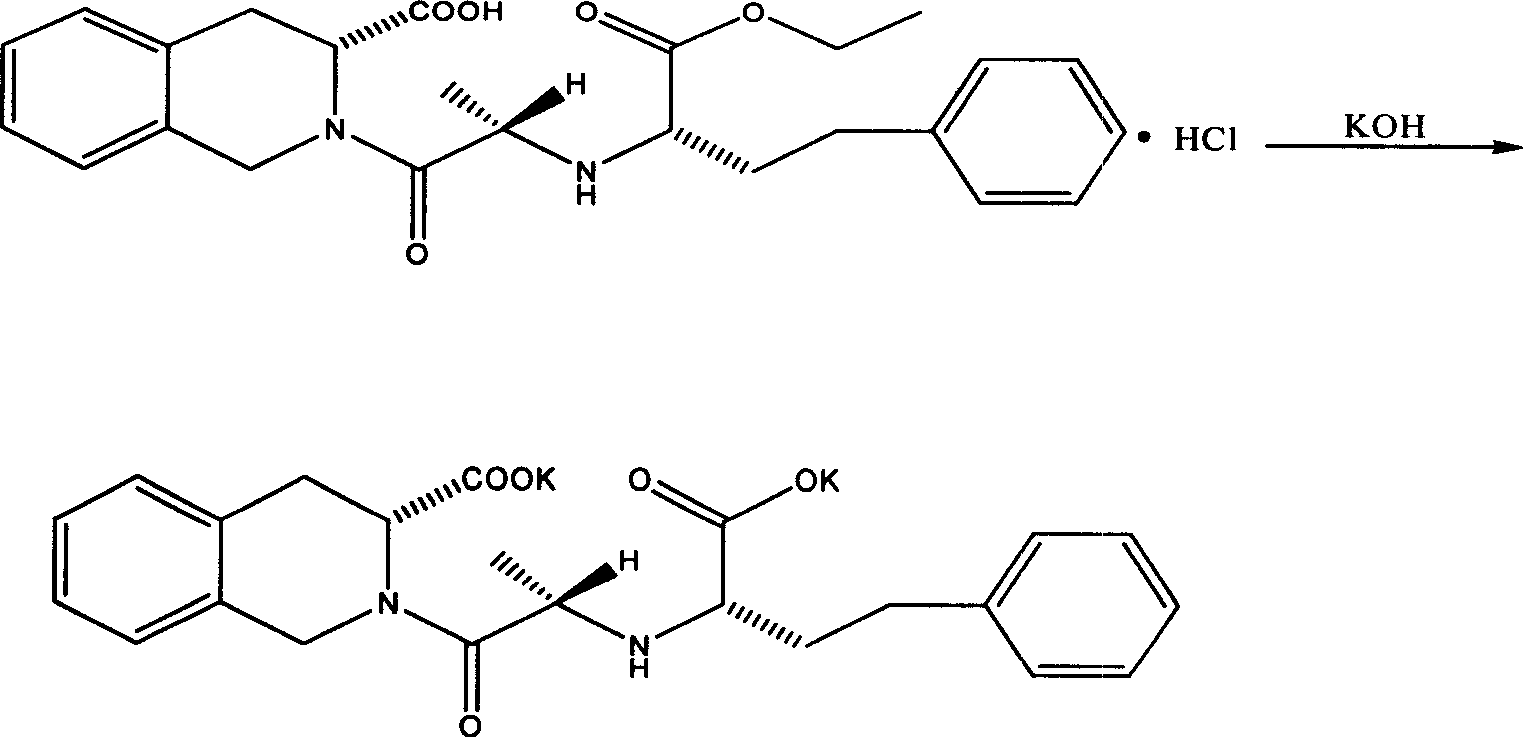
[0041] Embodiment 1 Preparation of quinaprilat metal salt
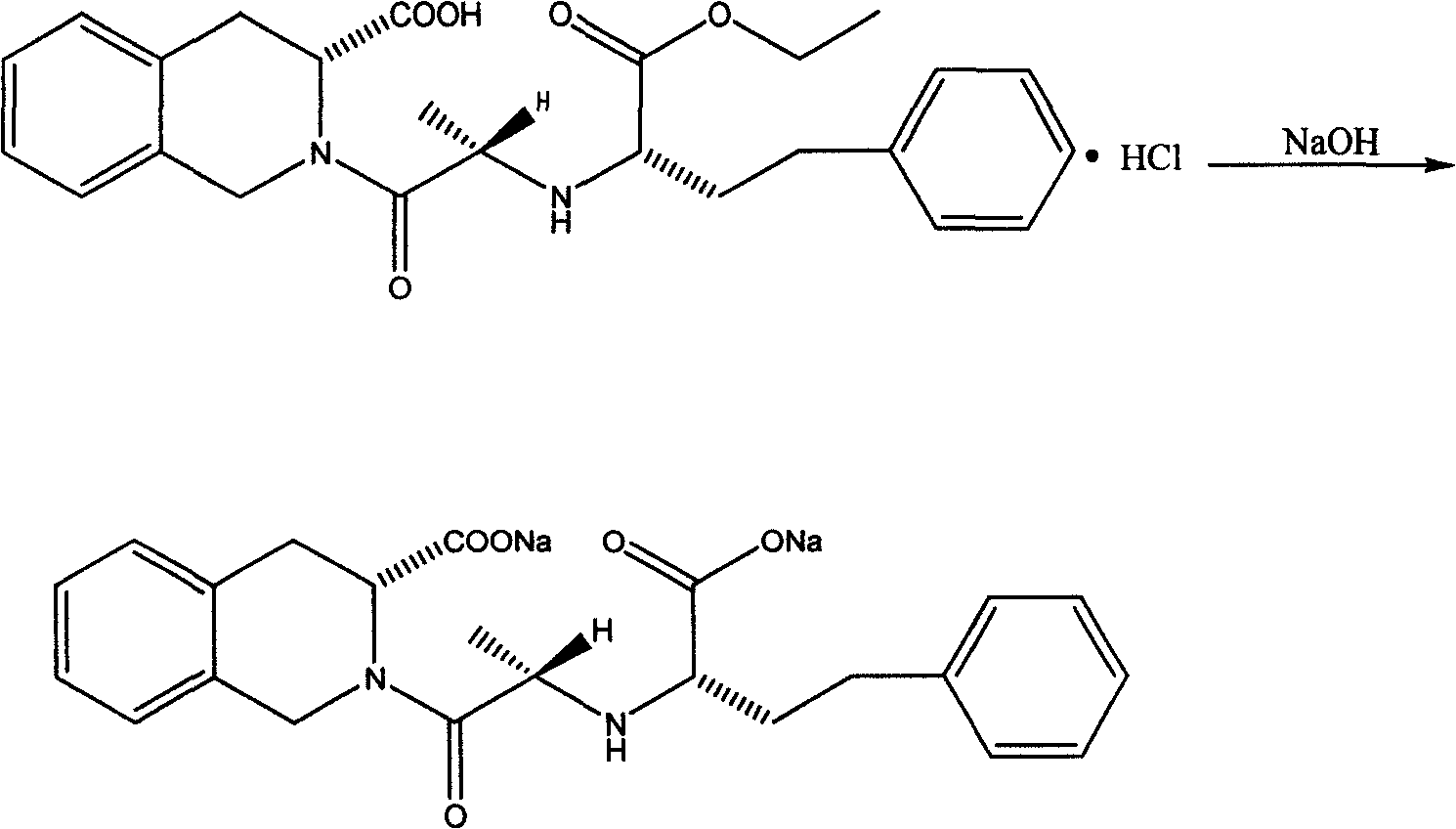
[0042] 1) preparation of quinaprilat sodium:
[0043] Reaction formula:
[0044]
[0045] Take 5.0 g of refined quinapril hydrochloride, dissolve it in 100 ml of dioxane:water (9:1), stir for 5 min, adjust the pH of the solution to 12 with 1N NaOH, and react at 60°C. After 20 minutes, add 1N NaOH to adjust the pH of the solution to 12, and continue to stir for 30 minutes to complete the reaction (can be monitored by spotting the plate). Add 1.0 g of activated carbon, filter while hot after 15 minutes, the filtrate is rotary steamed at 50°C for 1 hour and becomes an oily substance, add 100ml of acetone that has been preheated to boiling, shake vigorously, the oily substance dissolves, and the insoluble substance is NaCl, filter while hot. Put the filtrate in an environment of 0-5°C for about 3 hours, a large amount of white solid appeared, filter it, dry the solid in vacuum at 40°C for 20 hours, weigh about 4.0g,...
Embodiment 2
[0061] Embodiment 2 Preparation of aseptic powder of the present invention
[0062] 1. Prescription
[0063] Prescription 1:
[0064]
[0065] Prescription 2:
[0066]
[0067] Prescription 3
[0068]
[0069] 2. Preparation process:
[0070] In the aseptic batching room, the aseptic compound of the present invention is weighed according to the prescription, subpackaged and sealed to obtain the product.
Embodiment 3
[0071] Example 3 Preparation of the aqueous injection of the present invention
[0072] 1. Prescription
[0073] Prescription 1:
[0074]
[0075] Prescription 2:
[0076]
[0077] Prescription 3:
[0078]
[0079] 2. Preparation process:
[0080] 1) Pre-treat the containers, ampoules, production equipment, instruments, etc. used for liquid preparation.
[0081] 2) Weigh the raw materials and auxiliary materials according to the prescription quantity.
[0082] 3) Add the raw materials into water for injection with a dosing volume of 80%, stir and dissolve, add 0.1% activated carbon for needles, stir at 50°C for 10 minutes, and filter for decarbonization.
[0083] 4) Measure the pH value of the solution, add water to make up to the total volume.
[0084] 5) Filter with a 0.45 μm microporous membrane.
[0085] 6) Check the clarity of the solution.
[0086] 7) Inspection of semi-finished products.
[0087] 8) The semi-finished product is melted and sealed in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com