18F-Fallypride automatically synthesizing method
An automated synthesis, sep-pakc-18 technology, applied in the direction of organic chemistry methods, chemical instruments and methods, isotope introduction of organic compounds, etc., can solve the problems of long time-consuming high-efficiency liquid phase purification, unfavorable clinical promotion and use, expensive preparative columns, etc. problems, achieve the effect of high labeling rate, easy operation and high synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
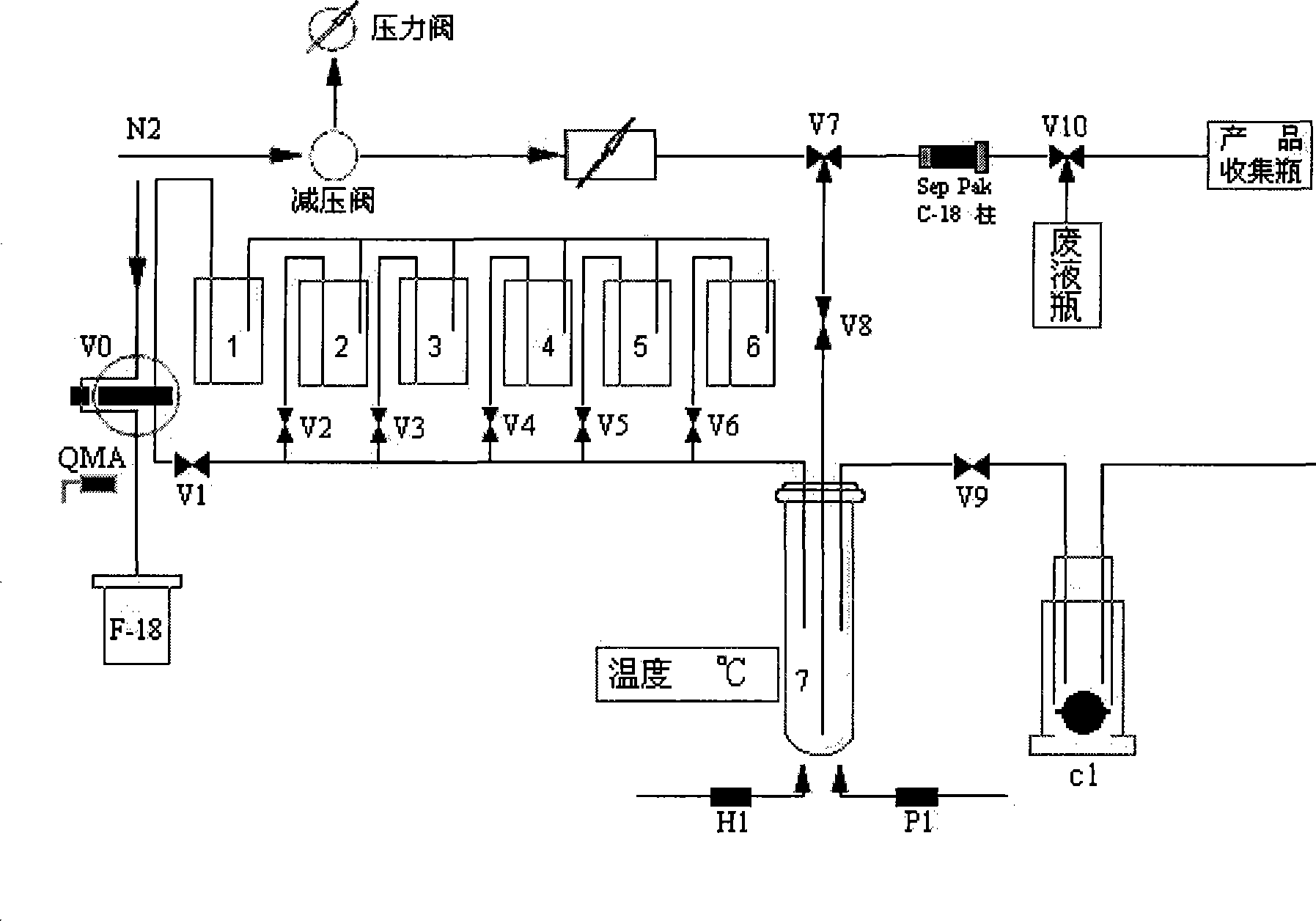
[0019] 18 The method of F-Fallypride automatic synthesis is carried out by using the domestic PET-MF-2V-IT-I multifunctional fluorine labeling module 18 Automated synthesis of F-Fallypride, synthesized using Sep-Pak C-18 column pair 18 F-Fallypride was purified:
[0020] Produced by the accelerator 18 The F-solution was absorbed by the QMA column and was absorbed by the 1.5mL K-containing solution in the bottle (1). 222 (50~300mg / mL) and K 2 CO 3 (2~8mg / mL) acetonitrile / water=10 / 1 (V / V) solution is eluted into the reaction tube (7), after heating at 115°C to remove acetonitrile, add 2 mL of acetonitrile in the bottle (2) to the reaction tube Azeotropic removal of water and acetonitrile, then add 4-5mg of 18 F-Fallypride labeled precursor, react at 90°C for 25 minutes, cool down, add 2 mL of 80% acetonitrile in the bottle (4) to mix with it, start the negative pressure to make the liquid in the reaction bottle pass through the tee behind the Sep-Pak C-18 column Into the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com