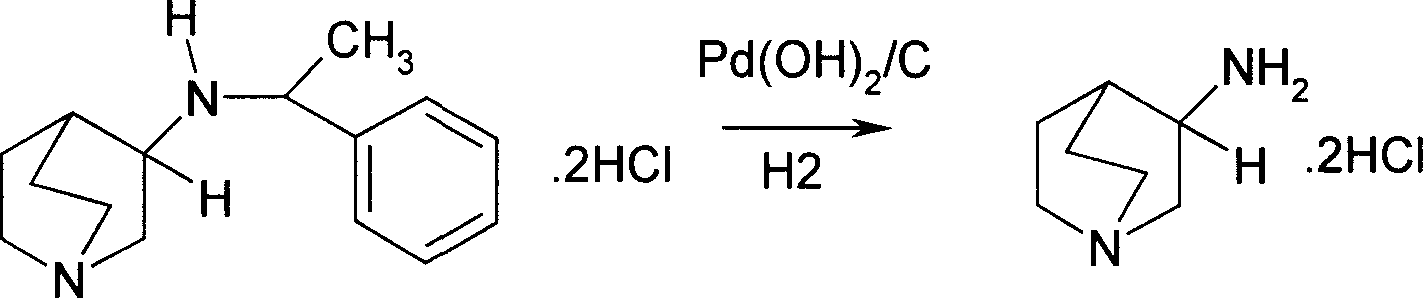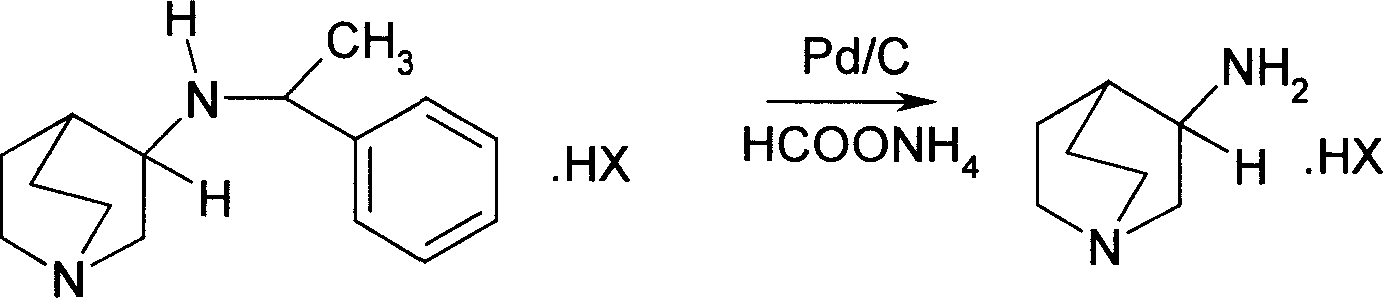Method for preparing 3-amido quinine medically-acceptable salt
A technology for pharmaceutically acceptable salts of aminoquinine, which is applied to the preparation of pharmaceutically acceptable salts of 3-aminoquinine, the key intermediate field, can solve the problems of high cost and high equipment requirements, and achieve mild conditions, high reaction yield, Post-processing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Hydrogenate 5g (0.016mol) of (3S)-3-[(R)-1-phenyl]-ethylamine]-quinine dihydrochloride, 2.08g (0.033mol) of ammonium formate and 10% palladium on carbon Catalyst 0.25g, 200ml methanol was added and heated at 60°C for 3 hours, the catalyst was filtered off after cooling, the target product (S)-3-aminoquinine dihydrochloride 2.79g was collected after the solvent was recovered, the yield was 85%. 1 HNMR(DMSO-d 6 )δ: 1.75~1.94(m,3H,), 2.15~2.22(m,1H), 232(q,1H), 3.11~3.20(m,5H), 3.55~3.68(m,2H); ESI(m / z): 126[M-2HCl]; (C=1, H 2 O); mp>285°C.
Embodiment 2
[0031] Hydrogenate 5g (0.016mol) of (3R)-3-[(S)-1-phenyl]-ethylamine]-quinine dihydrochloride, 3.12g (0.05mol) of ammonium formate and 5% palladium on carbon The catalyst was 1.5g, 200ml of tetrahydrofuran was added, and the reaction was carried out at 30°C for 8 hours. After cooling, the catalyst was filtered off. After the solvent was recovered, 2.62g of the target product (R)-3-aminoquinine dihydrochloride was collected, with a yield of 80%. 1 HNMR(DMSO-d 6 )δ: 1.75~1.93(m,3H,), 2.14~2.20(m,1H), 2.31(q,1H), 3.10~3.24(m,5H), 3.55~3.68(m,2H); EI-MS (m / z): 126[M-2HCl]; (C=1, H 2 O); mp>285°C.
Embodiment 3
[0033] Add 5g (0.013mol) of (3S)-3-[(S)-1-phenyl]-ethylamine]-quinine dihydrobromide, 4.0g (0.063mol) of ammonium formate and 10% palladium on carbon Hydrogen catalyst 1g was added with 200m of toluene, heated to reflux at 90°C for 5 hours, the catalyst was filtered off after cooling, the target product (S)-3-aminoquinine dihydrobromide 3.04g was collected after the solvent was recovered, and the yield was 83%. 1 HNMR(DMSO-d 6 )δ: 1.75~1.94(m,3H,), 2.15~2.22(m,1H), 2.32(q,1H), 3.11~3.20(m,5H), 3.55~3.68(m,2H); ESI(m / z): 126[M-2HCl]; mp>285°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com