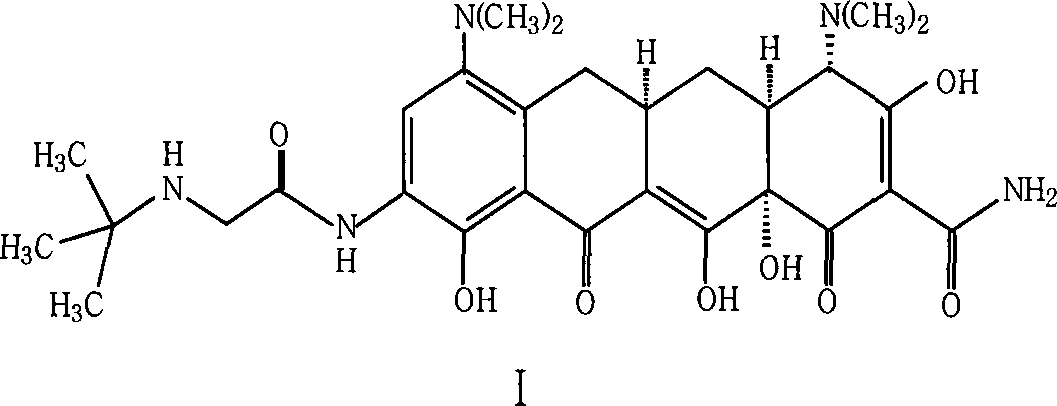
Method for preparing glycylcycline freezing-dried powder injection
A technology of tigecycline and freeze-dried powder injection, which is applied in the field of preparation of tigecycline, can solve the problems of low dosage of antioxidant efficiency, unworthy of use, potential safety hazards, etc., and achieves safer clinical medication and clinical medication. Safety and stability-enhancing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Take 2.5g of tigecycline, add about 80ml of water for injection (water temperature 20.4°C), stir to dissolve, adjust the pH value of the solution to 5.48 with 0.3mol / L hydrochloric acid solution, add water for injection to 100ml, shake well, add 100mg (0.1% volume of water for injection) of activated carbon for injection, stirred for about 30 minutes, coarsely filtered and decarbonized, then filtered through 0.45 μm and 0.22 μm microporous membranes respectively, and distributed in 10ml controlled antibiotic bottles, the filling volume is 2.0ml / bottle, after pre-freezing for 4 hours, put it into a freeze dryer for 36 hours to freeze-dry, press the cap, and tie the aluminum cap to get this product.
Embodiment 2
[0024] Example 2 Take 2.5g of tigecycline, add about 80ml of water for injection (water temperature 20.4°C), stir to dissolve, adjust the pH value of the solution to 5.46 with 0.2mol / L sulfuric acid solution, add water for injection to 100ml, shake well, add 100mg (0.1% volume of water for injection) of activated carbon for injection, stirred for about 30 minutes, coarsely filtered and decarbonized, then filtered through 0.45 μm and 0.22 μm microporous membranes respectively, and distributed in 10ml controlled antibiotic bottles, the filling volume is 2.0ml / bottle, after pre-freezing for 4 hours, put it into a freeze dryer for 36 hours to freeze-dry, press the cap, and tie the aluminum cap to get this product.
Embodiment 3
[0025] Example 3 Take 2.5g of tigecycline, add about 80ml of water for injection (water temperature 20.4°C), stir to dissolve, adjust the pH value of the solution to 5.40 with 0.5mol / L tartaric acid solution, add water for injection to 100ml, shake well, add 100mg (0.1% volume of water for injection) of activated carbon for injection, stirred for about 30 minutes, coarsely filtered and decarbonized, then filtered through 0.45 μm and 0.22 μm microporous membranes respectively, and distributed in 10ml controlled antibiotic bottles, the filling volume is 2.0ml / bottle, after pre-freezing for 4 hours, put it into a freeze dryer for 36 hours to freeze-dry, press the cap, and tie the aluminum cap to get this product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com