Fipronil, ethiprole and synthesizing method for derivative thereof
A synthetic method and technology of trifluoromethylphenyl, applied in the field of synthesizing 1-phenyl-3-cyano-5-aminopyrazole derivatives, can solve the problem of low yield, complex operation and treatment, and inability to realize industrial production And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
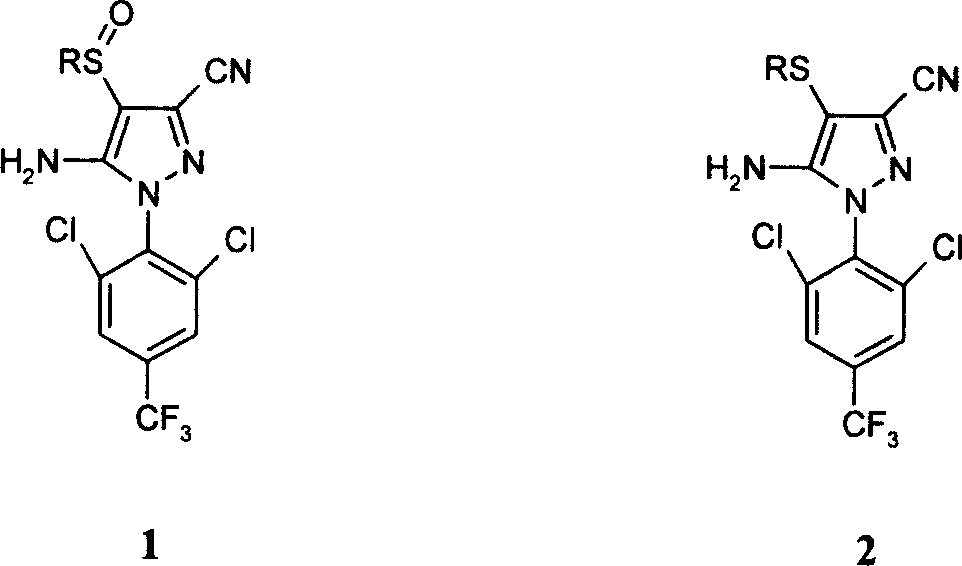
[0026] Preparation of 1-(2,6-dichloro-4-trifluoromethylphenyl)-3-cyano-4-trifluoromethanesulfinyl-5-amino-pyrazole
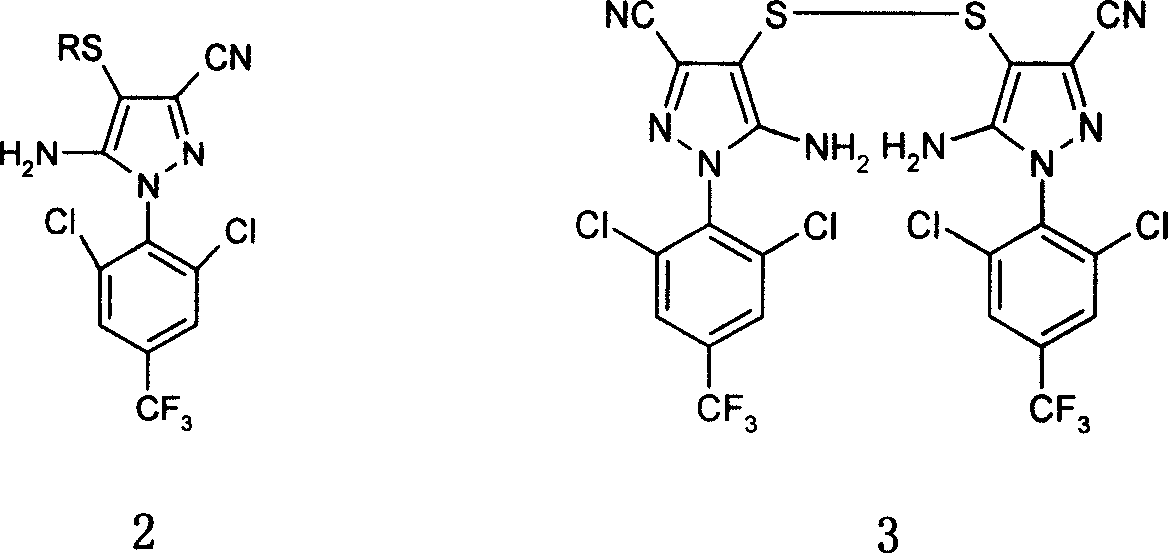
[0027] In ionic liquid [Bmim]PF 6(20mL) and acetonitrile (20mL), add 5-amino-1-(2,6-dichloro-4-trifluoromethylphenyl)-3-cyano-4-trifluoromethylthio Pyrazole (2.15g, 5mmol), ruthenium trichloride (9.6mg, 0.05mmol), pyridine (1mL), add acetonitrile solution (10mL) containing trichloroisocyanuric acid (1.16g, 5mmol) dropwise, 20 minutes Finish. React at 40~50℃ for 1 hour, after the reaction is over, use NaHSO at 20℃ 3 The excess trichloroisocyanuric acid was quenched, ice water (200 mL) was added and stirred, the product was filtered, the ionic liquid was recovered and reused, and the colorless target product was obtained by column chromatography with a yield of 71% and no oxidation by-products.
[0028] The following are the physical data and detection data of 1-(2,6-Dichloro-4-trifluoromethylphenyl)-3-cyano-4-trifluoromethanesulfinyl-5-amino-pyrazole.
[0029] Colorl...
Embodiment 2
[0035] The preparation reaction substrate of 1-(2,6-dichloro-4-trifluoromethylphenyl)-3-cyano-4-ethylsulfinyl-5-amino-pyrazole was changed to 1-(2 ,6-Dichloro-4-trifluoromethylphenyl)-3-cyano-4-ethylmercapto-5-amino-pyrazole, the synthesis method is similar to that in Example 1, and the yield is 75%
[0036] The following are the physical and detection data of 1-(2,6-Dichloro-4-trifluoromethylphenyl)-3-cyano-4-ethylsulfinyl-5-amino-pyrazole.
[0037] Colorless crystal, melting point: 179-181℃
[0038] IR (KBr): 3423, 3336, 3240, 2251, 1634, 1568, 1400, 1319, 1135, 875, 818cm -1 .
[0039] 1 H NMR(300MHz, C 3 D 6 O): δ = 8.12 (s, 2H), 6.36 (s, 2H), 3.21 (q, J = 7.4 Hz, 2H), 1.29 (t, J = 7.4 Hz, 3H).
[0040] 13 C NMR(75MHz, C 3 D 6 O): δ=7.0(1C), 51.5(1C), 100.0(1C), 111.2(1C), 122.4(q, J=271.5Hz, 1C), 126.3(1C), 126.6(2C), 126.7(2C) ), 134.4(q, J=34.1Hz, 1C), 134.8(1C), 136.6(1C).
[0041] 19 F NMR(220MHz, C 3 D 6 O): δ=-63.6(s, 3F).
[0042] In the same way, replace the substrate...
Embodiment 3
[0053] K 2 CO 3 (0.69g, 5mmol) was added to containing 1-(2,6-dichloro-4-trifluoromethyl)phenyl-3-cyano-5-amino-4-pyrazolyl disulfide (2g, 2.5 In a solution of DMF (30ml) in mmol), ethyl iodide (6mmol) was injected into the reaction mixture, water (0.5ml) was injected, and then HOCH was added. 2 SO 2 Na(1.18g, 10mmol), react at room temperature for 1 hour, pour into ice water and stir for 20 minutes, filter to obtain 1-(2,6-dichloro-4-trifluoromethylphenyl)-3-cyano-4 -Ethylmercapto-5-aminopyrazole, the yield is 95%.
[0054] Melting point: 150~151℃.
[0055] IR (KBr): 3456, 3320, 3220, 2925, 2252, 1625, 1554, 1392, 1314, 1146, 876, 811cm -1 .
[0056] 1 H NMR(300MHz, C 3 D 6 O): δ = 8.08 (s, 2H), 6.03 (s, 2H), 2.30 (s, 3H).
[0057] 13 C NMR(75MHz, C 3 D 6 O): δ=18.9(1C), 95.1(1C), 122.5(q, J=271.1Hz, 1C), 126.38(2C), 126.42(2C), 131.4(1C), 133.7(q, J=34.0Hz , 1C), 136.5(1C), 151.2(1C).
[0058] 19 F NMR(220MHz, C 3 D 6 O): δ=-63.6(s, 3F).
[0059] Replacing sodium hydroxymethanes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com