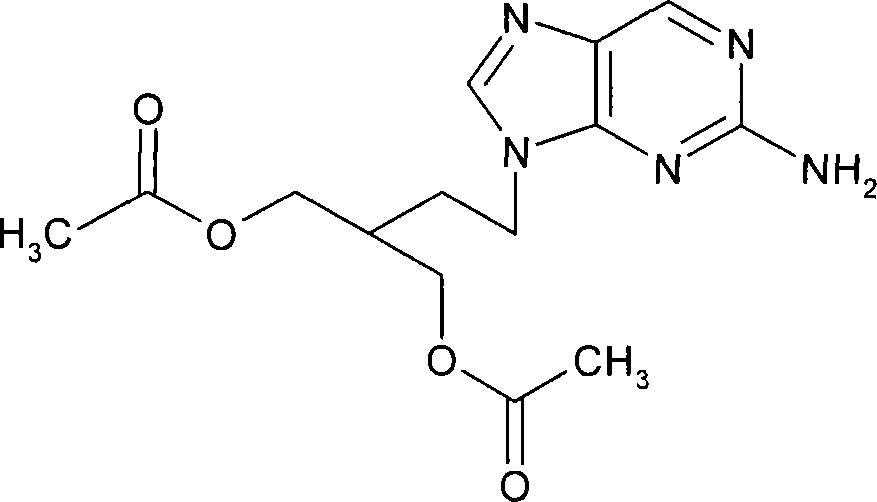
Modified release famciclovir pharmaceutical compositions
A technology of famciclovir and composition, which is applied in the field of composition for improved release, and can solve problems such as not disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The preparation of the pharmaceutical compositions of the present invention begins by mixing the therapeutic compound with a release retardant to form an extrudate using melt granulation. The release retardant may, for example, be present in an amount of from about 5% to about 40% by weight of the extrudate composition, such as from about 10% to about 35%, such as from about 25% to about 30%. Similarly, the therapeutic compound may be present in an amount of from about 60% to about 99% by weight of the extrudate composition, such as from about 70% to about 90%, such as from about 80% to about 85%.
[0048] For example the extrudate is subsequently ground to form granules which form the internal phase of the pharmaceutical composition. One of ordinary skill in the art will know the necessary particle size of the particles required to formulate a particular pharmaceutical composition. For example, suitable particle sizes include those of 1000 μm, 750 μm, 500 μm or 250 μm...
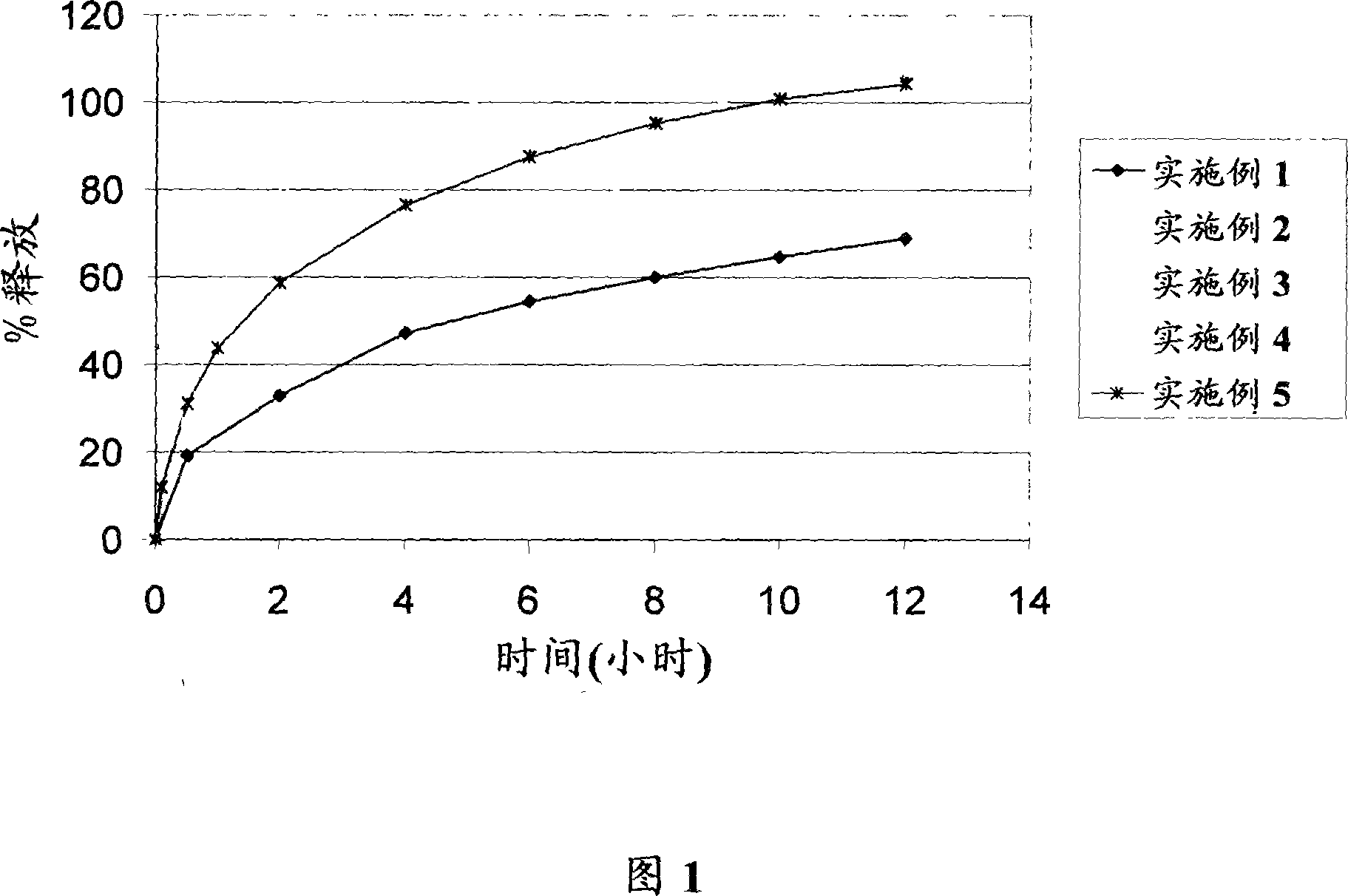
Embodiment 1
[0065] Internal phase ingredients: Famciclovir, PVA / PVP mixture (commercially available from BASF AG (Ludwigshafen, Germany) as KOLLIDON SR) and silicon dioxide were sieved through a #18 mesh sieve (i.e. 1 mm sieve) and a premix was prepared . The internal phase is then introduced into the feed section or funnel of the twin-screw extruder. A suitable twin-screw extruder is a PRISM 16 mm pharmaceutical twin-screw extruder commercially available from Thermo Electron Corp (Waltham, Massachusetts).
[0066] A twin-screw extruder is equipped with 4 separate barrel zones or sections and does not have a 5th zone (i.e. die). Starting from the funnel, the zones were heated to the following temperatures: 90°C, 90°C, 60°C and 40°C, respectively. The speed of the shaft was gradually increased to 150 rpm as the material was processed through the extruder.
[0067] The extrudate or pellets obtained from the extruder are then cooled to room temperature. After cooling the extrudate...
Embodiment 2
[0070] Element
[0071] Example 2 was prepared using the same method disclosed in Example 1, however, the concentrations of the ingredients were different.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com