Trimethine dimer compound and optical recording medium using same
A technology of methyl dimer and compound, applied in the direction of optical record carrier, temperature recording method, record carrier material, etc., can solve the problems of no record of high-speed recording characteristics, no record of high-speed recording, restrictions on substituents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
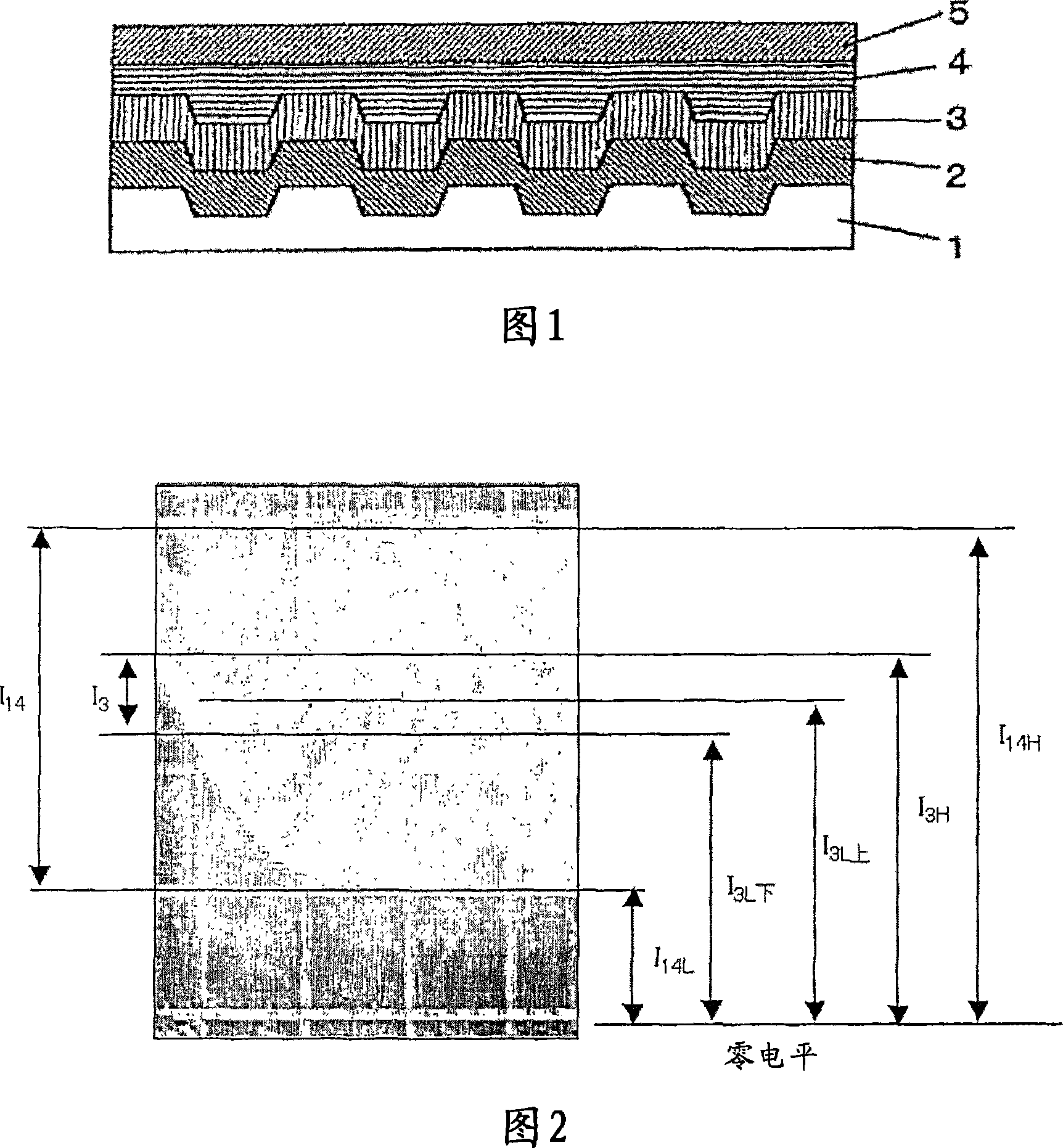
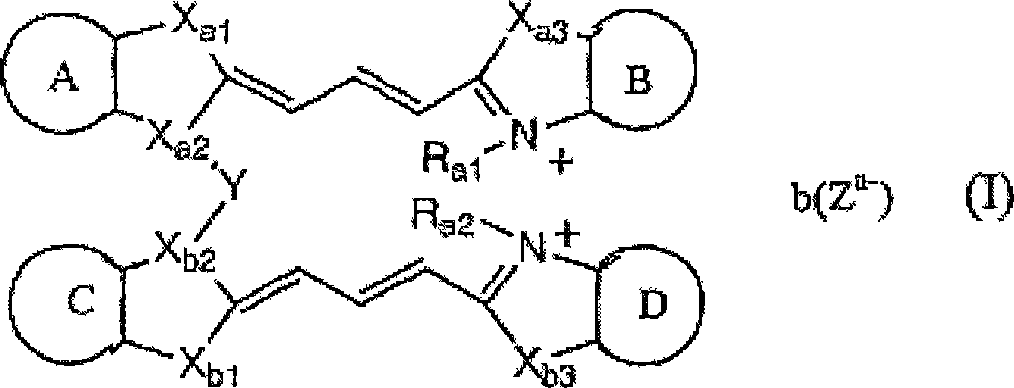
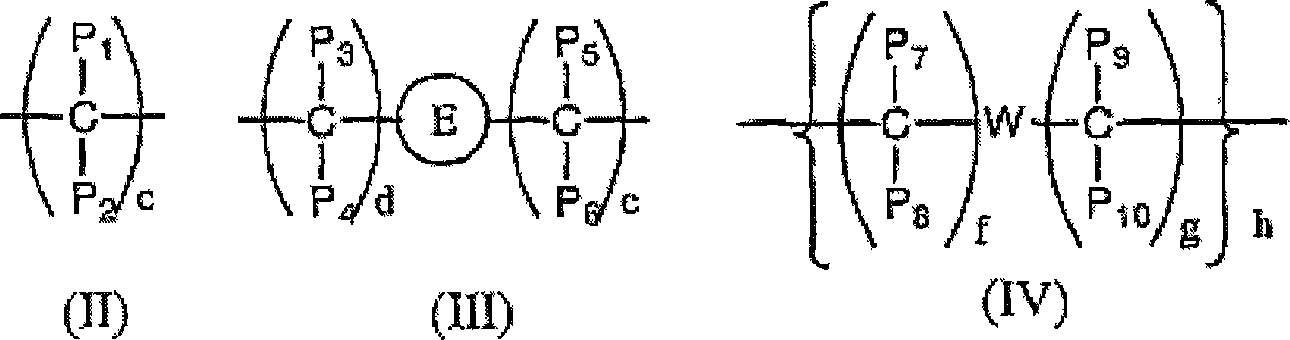
Image
Examples
Synthetic example 1
[0391] [Synthesis example 1] Synthesis of indolene compound (A)
[0392] Indoles (A)
[0393] In a nitrogen atmosphere, 115.1 g of the compound represented by the following structural formula ① and 69.1 g of α,α'-dibromo-p-xylene were added to 500 mL of toluene, and the mixture was stirred under reflux for 19 hours. After cooling, the precipitate was collected by filtration, and after washing with toluene, the filtered substance was dissolved in 500 mL of methanol, and the insoluble matter was filtered off. 23.1 g of sodium hydroxide was added to the methanol filtrate, and after stirring for 30 minutes at room temperature, the precipitate was collected by filtration. The filtered material was washed with water and toluene, and dried in vacuum to obtain 113.3 g of white powder of the indolenine compound (A) decomposed at 236°C.
[0394]
[0395] The element analysis value and mass spectrum analysis value of this compound are shown below.
[0396] Elemental analysis value (C 38 ...
Synthetic example 2
[0401] [Synthesis example 2] Synthesis of indolene compound (B)
[0402] Indoles (B)
[0403] In a nitrogen atmosphere, 115.1 g of the compound represented by the following structural formula ① and 82.2 g of 1,5-bis-bromomethylnaphthalene were added to 500 mL of toluene, and the mixture was stirred for 19 hours under reflux. After cooling, the precipitate was collected by filtration, and after washing with toluene, the filtered substance was dissolved in 500 mL of methanol, and the insoluble matter was filtered off. 33 g of sodium hydroxide was added to the methanol filtrate, and after stirring at room temperature for 30 minutes, the precipitate was collected by filtration. The filtrate was washed with water and toluene, and then dried in vacuum to obtain 93 g of a white powdery indole compound (B) with a melting point of 117 to 120° C. (yield 65%).
[0404]
[0405] The element analysis value and mass spectrum analysis value of this compound are shown below.
[0406] Elementa...
Embodiment 1
[0411] [Example 1] Preparation of a tertiary methyl dimer compound (specific example compound (A-11) in Table 1)
[0412] Specific example compound (A-11)
[0413] In a nitrogen atmosphere, 5.2 g of the indolenine compound (A) synthesized in Synthesis Example 1 and 7.3 g of the compound represented by the following structural formula ② were added to 50 mL of acetic anhydride, and after stirring at room temperature for 10 minutes, 2 g of methanesulfonic acid was added. Stir at 83~86°C for 2.0 hours. Cool, add 100 mL methanol dropwise, and stir under reflux for 1 hour. After cooling, methanol was concentrated under reduced pressure, 150 mL of methanol was added to the residue, 3.16 g of 70% perchloric acid aqueous solution was added dropwise, and the mixture was stirred at room temperature for 1 hour. The precipitate was collected by filtration, washed with methanol, and dried to obtain 13.5 g (yield: 95.3%) of the specific example compound (A-11) of Table 1 as red-purple crystals...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com