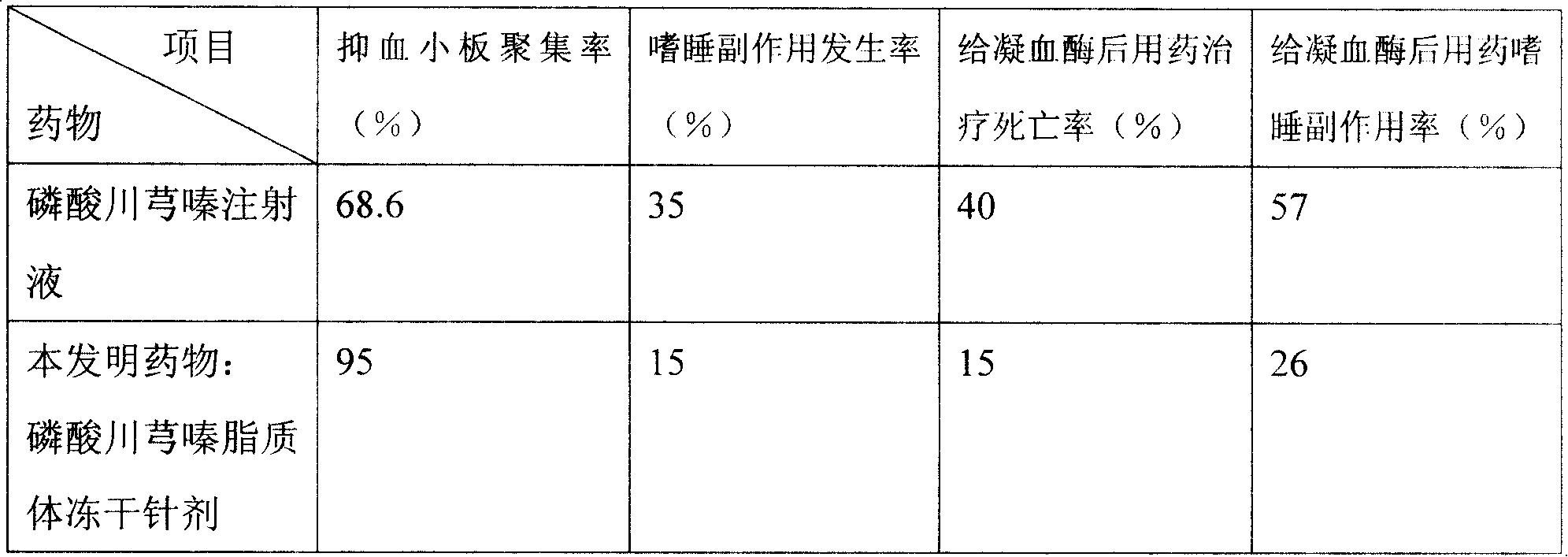
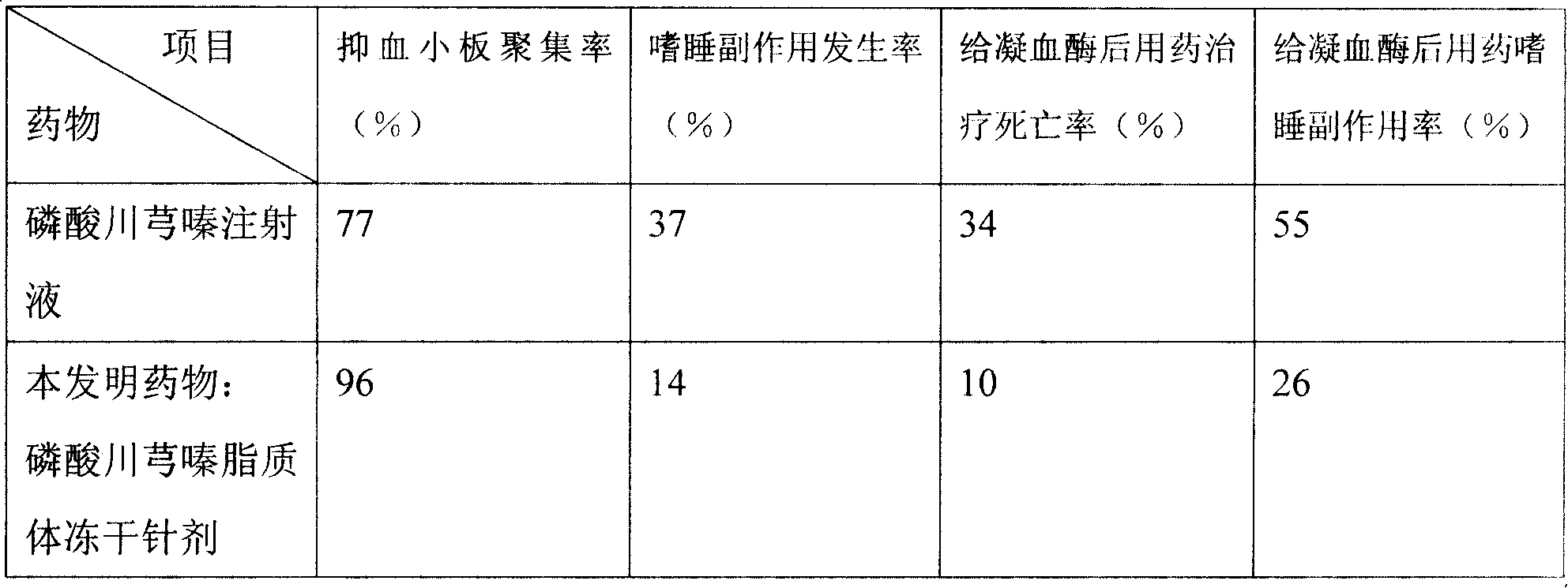
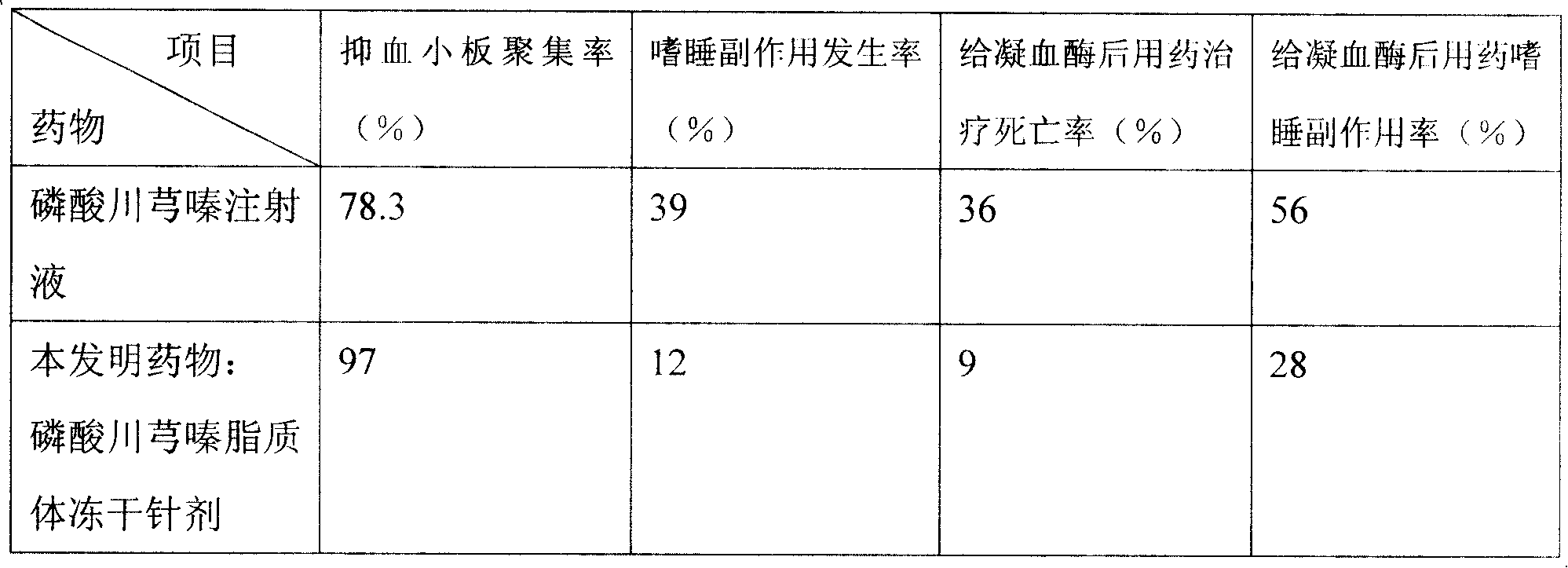
Ligustrazine microcosmic salt liposome medicine and preparing method
Ligustrazine phosphate lipid and liposome technology, which can be used in liposome delivery, drug combination, pharmaceutical formula, etc., can solve the problem of weak ability of anti-oxidation in vitro and peroxidation in vivo, obvious side effects of digestive system, and problems for patients and patients. Inconvenience for doctors and other problems, achieve the effect of reducing the number of medications, improving the curative effect, and resisting allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The formula adopted in the present embodiment is as follows (consumption unit: gram):
[0031] Egg yolk lecithin, sheep brain lecithin 4:1 mixture by weight 200;
[0032] Stigmasterol and campesterol weight ratio 3: 1 mixture 170;
[0033] Reduced Glutathione 2.5;
[0034] Ligustrazine Phosphate 15;
[0035] tert-butanol, absolute ethanol, acetone weight ratio 1: 0.01: 0.02 mixture 720;
[0036] Polyethylene glycol-2000, Tween 801 weight ratio 4: 0.5 mixture 140;
[0037] Hypromellose 100.
[0038] In this embodiment, 1700 grams of 0.01 M phosphate buffer solution with a pH of 3.0-7.5 is used.
[0039] The preparation method of the present embodiment is as follows:
[0040] (1) egg yolk lecithin, sheep brain lecithin, stigmasterol, and campesterol are dissolved in the tert-butanol, dehydrated alcohol and acetone mixed solution of the amount described, fully dissolved completely;
[0041] (2) Add the amount of reduced glutathione, ligustrazine phosphate, polyethyl...
Embodiment 2
[0056] The formula adopted in the present embodiment is as follows (consumption unit: gram):
[0057] Egg yolk lecithin, sheep brain lecithin 4:1 weight ratio mixture 260;
[0058] Stigmasterol and campesterol weight ratio 3: 1 mixture 200;
[0059]Reduced Glutathione 6;
[0060] Ligustrazine Phosphate 20;
[0061] tert-butanol, absolute ethanol, acetone weight ratio 1: 0.01: 0.07 mixture 1000;
[0062] Polyethylene glycol-2000, Tween 801 weight ratio 4: 1 mixture 150;
[0063] Hypromellose 110.
[0064] In this embodiment, 2500 grams of 0.01 M phosphate buffer solution with pH 3.0-7.5 is used.
[0065] The preparation method of the present embodiment is as follows:
[0066] (1) egg yolk lecithin, sheep brain lecithin, stigmasterol, and campesterol are dissolved in the tert-butanol, dehydrated alcohol and acetone mixed solution of the amount described, fully dissolved completely;
[0067] (2) Add the amount of reduced glutathione, ligustrazine phosphate, polyethylene gl...
Embodiment 3
[0082] The formula adopted in the present embodiment is as follows (consumption unit: gram):
[0083] Egg yolk lecithin, sheep brain lecithin 4:1 mixture by weight 330;
[0084] Stigmasterol and campesterol weight ratio 3: 1 mixture 280;
[0085] Reduced Glutathione 10;
[0086] Ligustrazine Phosphate 25;
[0087] tert-butanol, absolute ethanol, acetone weight ratio 1: 0.05: 0.027 mixture 1200;
[0088] Polyethylene glycol-2000, Tween 801 weight ratio 4: 1 mixture 160;
[0089] Hypromellose 120.
[0090] In this embodiment, 2800 grams of 0.01 M phosphate buffer solution with a pH of 3.0-7.5 is used.
[0091] The preparation method of the present embodiment is as follows:
[0092] (1) egg yolk lecithin, sheep brain lecithin, stigmasterol, and campesterol are dissolved in the tert-butanol, dehydrated alcohol and acetone mixed solution of the amount described, fully dissolved completely;
[0093] (2) Add the amount of reduced glutathione, ligustrazine phosphate, polyethyle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com