Preparation of kilogram-grade scale high-purity monosialotetrahexosylganglioside
A ganglioside and monosialic acid technology, applied in the field of biomedicine, can solve the problems of difficulty in extraction and separation of high-purity GM1, no reports and applications, and difficulty in large-scale clinical application. Increased yield and production efficiency, low product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
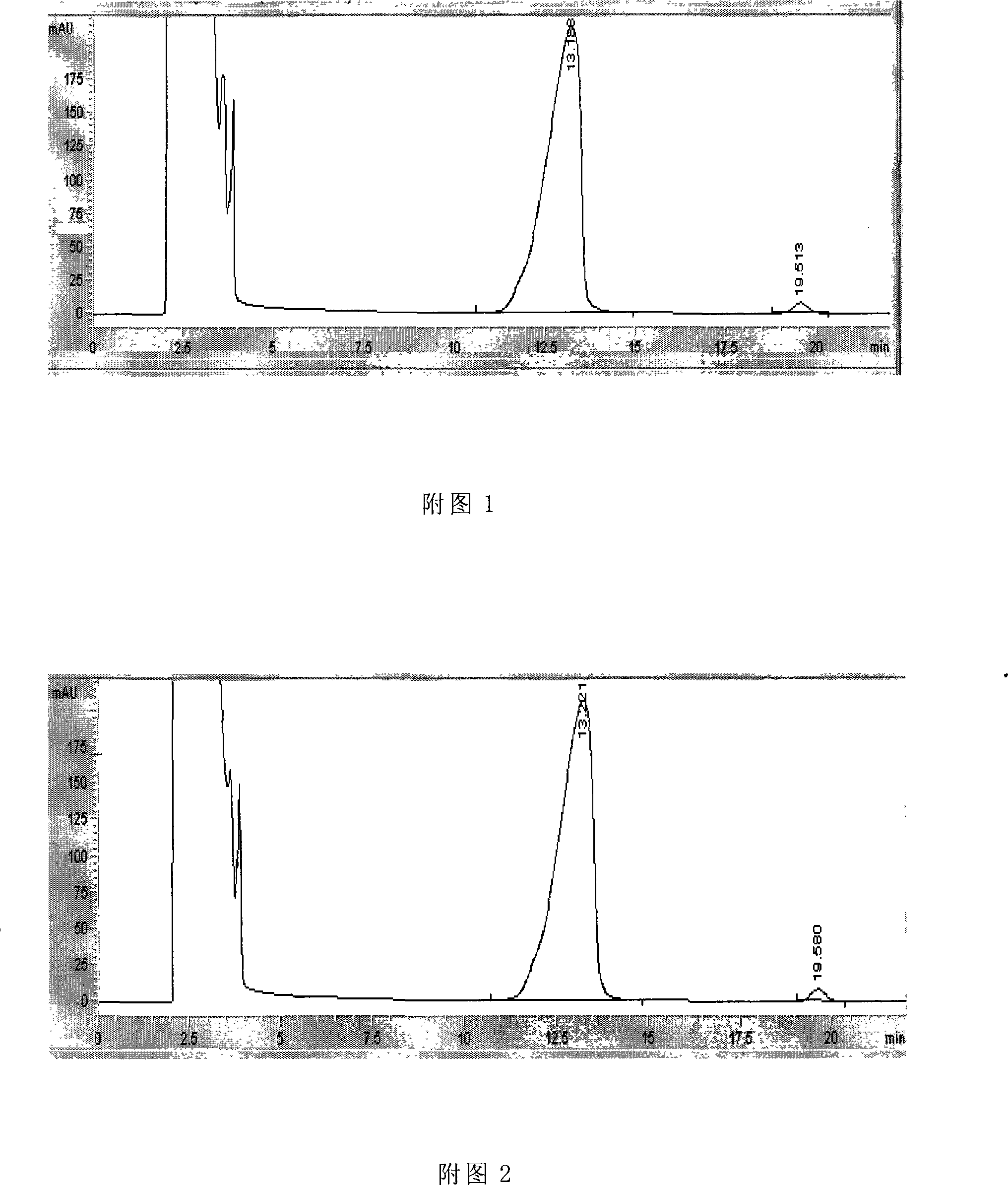
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of high-purity GM1 freeze-dried powder
[0024] 1) Centrifuge the fresh pig brain tissue at 5000rpm to remove free water, pulverize it with a colloid mill, homogenize it at a high speed of 3000r / min for 30min, and pour it into the extraction tank and add 10 times the volume of methanol water (2:0.8) solution , stirred at a low temperature of 2°C for 12 hours, and extracted;
[0025] 2) Use a plate and frame filter to achieve solid-liquid separation of the extract, and then apply it to a macroporous adsorption resin D-101 chromatographic column, first wash off impurities with low-concentration methanol water (1:2), and then elute with anhydrous methanol Collecting the eluate containing gangliosides;
[0026] 3) The ganglioside eluate was concentrated in vacuum at 40°C and freeze-dried at -20°C, dissolved in 0.5M NaCl solution and adjusted to pH 3.5 with glacial acetic acid, hydrolyzed at 60°C for 3 hours, and used continuously during the hydrolysis...
Embodiment 2
[0030] Example 2 Preparation of high-purity GM1 freeze-dried powder
[0031]1) Centrifuge the fresh pig brain tissue at 5000rpm to remove free water, pulverize it with a colloid mill, homogenize it at a high speed of 3000r / min for 30min, and pour it into the extraction tank and add 10 times the volume of methanol water (2:1) solution , stirred at a low temperature of 8°C for 24 hours, and extracted;
[0032] 2) Use a plate and frame filter to achieve solid-liquid separation of the extract, and then apply it to a macroporous adsorption resin D-101 chromatographic column, first wash off impurities with low-concentration methanol water (1:3), and then elute with anhydrous methanol Collecting the eluate containing gangliosides;
[0033] 3) The ganglioside eluate was concentrated in vacuum at 80°C and freeze-dried at -60°C, dissolved in 0.5M NaCl solution and adjusted to pH 4.0 with glacial acetic acid, hydrolyzed at 75°C for 5 hours, and used continuously during the hydrolysis pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com