Method for preparing nano-grade rutile phase RuO2-SnO2 oxide
A rutile phase, ruo2-sno2 technology, applied in the field of electrochemistry, can solve the problems of unsatisfactory comprehensive performance of titanium anodes and difficulties in technology promotion, and achieve the effect of convenient operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
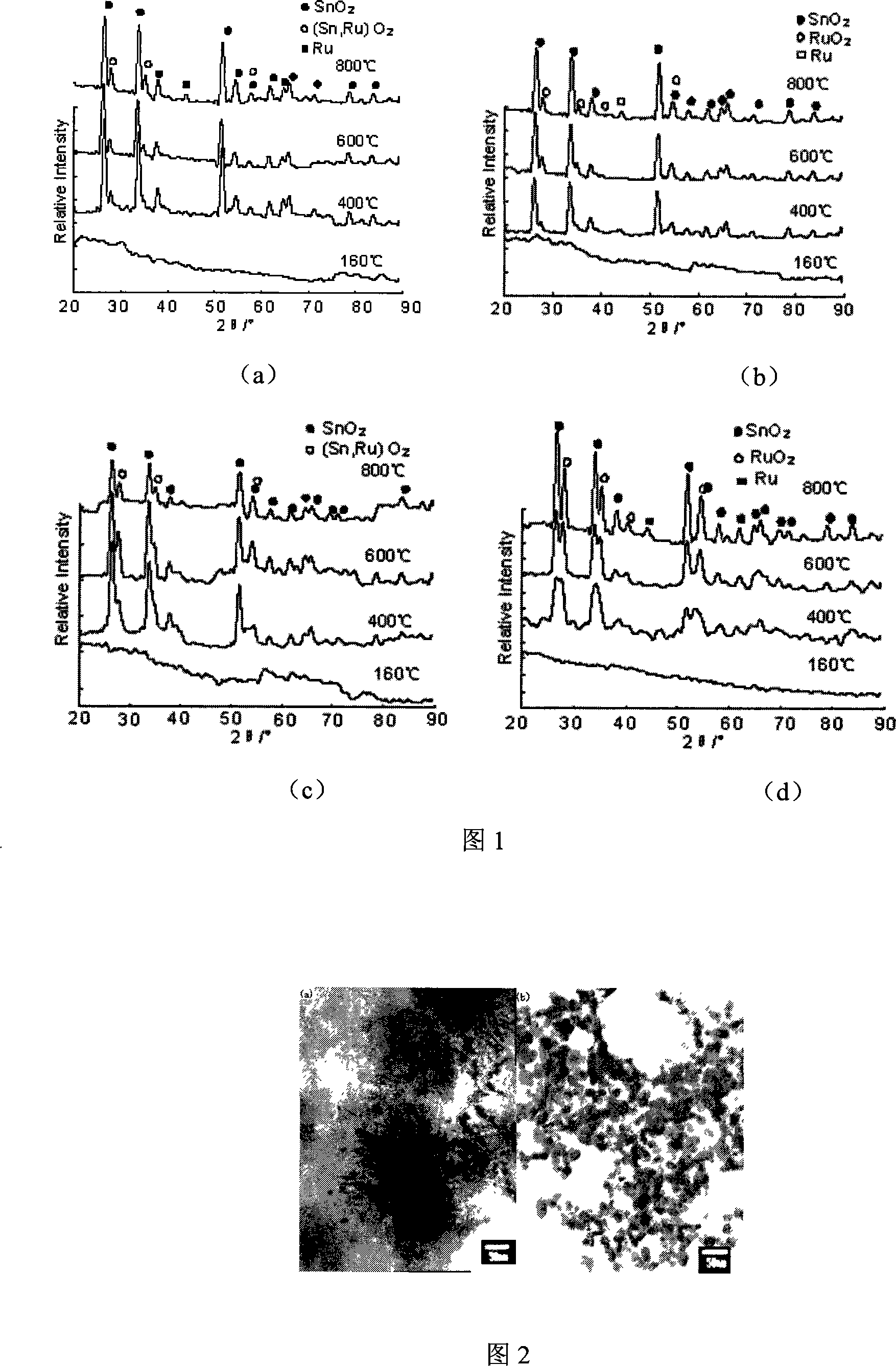
[0024] Weigh 1mol of SnCl 4 ·6H 2 O was completely dissolved in absolute ethanol, 3 mol of citric acid was weighed and completely dissolved in ethanol, and then SnCl 4 The ethanol solution was added dropwise to the citric acid ethanol solution to form a mixed solution and stirred thoroughly; 1moRuCl was weighed 3 ·3H 2 Put O into a beaker, immediately dilute it with ethanol until it is completely dissolved, heat and stir, weigh 3mol of citric acid and dissolve it in ethanol completely, then dissolve RuCl 3 The ethanol solution was dropped into the mixed solution of citric acid and ethanol at a rate of 30 drops / min, stirred thoroughly, and kept at 60° C. for more than 3 hours. The SnO formed above 2 and RuO 2 The solution was mixed according to the required molar ratio (see Table 1), continued to stir for more than 3 hours, and stood for 24 hours. The above coating solution was dried at 80°C and sintered at 400°C for 1 h. The structure size and specific surface area of ...
Embodiment 2
[0029] Weigh 1mol of SnCl 2 ·2H 2 O was completely dissolved in absolute ethanol, 3 mol of citric acid was weighed and completely dissolved in ethanol, and then SnCl 2 The ethanol solution was added dropwise to the citric acid ethanol solution to form a mixed solution and stirred thoroughly; 1 mol RuCl was weighed 3 ·3H 2 Put O into a beaker, immediately dilute it with an appropriate amount of ethanol until it is completely dissolved, heat and stir, weigh 3 mol of citric acid and dissolve it in ethanol completely, then dissolve the RuCl 3 The ethanol solution was dropped into the mixed solution of citric acid and ethanol at a rate of 30 drops / min, stirred thoroughly, and kept at 60° C. for more than 3 hours. The above is formed into SnO 2 and RuO 2 The solution was mixed according to the required molar ratio (see Table 2), continued stirring for more than 3 hours, and allowed to stand for 24 hours. The above coating solution was dried at 80°C and sintered at 400°C for 1 ...
Embodiment 3
[0033] Weigh 0.5mol of SnCl 2 ·2H 2 O was completely dissolved in absolute ethanol, 1.5mol of citric acid was weighed and completely dissolved in ethanol, and then SnCl 2 The ethanol solution was added dropwise to the citric acid ethanol solution to form a mixed solution and stirred well; 0.5mol of SnCl was weighed 4 ·6H 2 O was completely dissolved in absolute ethanol, 1.5mol of citric acid was weighed and completely dissolved in ethanol, and then SnCl 4 The ethanol solution was added dropwise to the citric acid ethanol solution to form a mixed solution and stirred thoroughly; 1 mol RuCl was weighed 3 ·3H 2 Put O into a beaker, immediately dilute it with an appropriate amount of ethanol until it is completely dissolved, heat and stir, weigh 3 mol of citric acid and dissolve it in ethanol completely, then dissolve the RuCl 3 The ethanol solution was dropped into the mixed solution of citric acid and ethanol at a rate of 30 drops / min, stirred thoroughly, and kept at 60° C....
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com