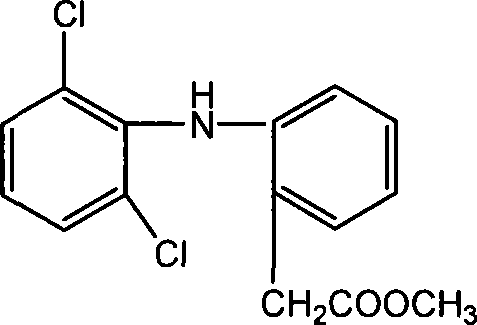
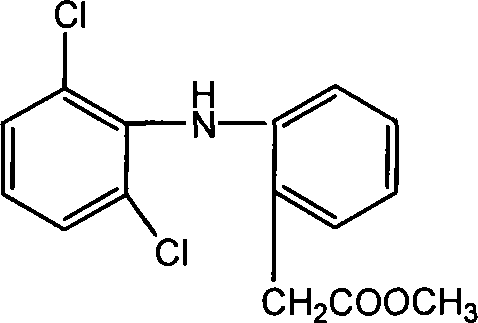
Methyl 2-(2-(2,6-dichlorophenylamino)phenyl)acetate and its synthesizing method and application
A technology of dichlorophenylamino and methyl acetate, which is applied in the direction of chemical instruments and methods, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as unusable, high toxicity and side effects, and large damage, and achieve Effect with low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Get diclofenac potassium (sodium) and dissolve it in water and acidify to obtain 14 g (70%) of diclofenac refined product. (mp is 156-158°C). 21.3g (188.4mmol) of chloroacetyl chloride was added dropwise under ice-cooling, and after the reaction was completed, 12.3g (88.6%) of methyl 2-(2-(2,6-dichlorophenylamino)phenyl)acetate was obtained, as shown below .
[0050]
Embodiment 2
[0052] 2 g (6.8 mmol) of diclofenac and 3.1 ml (76.4 mmol) of anhydrous methanol were sequentially added into the four-necked flask, and heated to reflux for 3 h. Spin-dry to obtain 1.9 g (90.0%) of methyl 2-(2-(2,6-dichlorophenylamino)phenyl)acetate, as shown below.
[0053]
Embodiment 3
[0055] Recipe quantity: 60g of methyl 2-(2-(2,6-dichlorophenylamino)phenyl)acetate, 84g of HPMCE4CR, 60g of anhydrous citric acid, 145.3g of lactose, 0.7g of magnesium stearate, a total of 1000 piece.
[0056] Preparation method: Take 2-(2-(2,6-dichlorophenylamino)phenyl)methyl acetate, HPMCE4CR, anhydrous citric acid, lactose and lubricant, pass through a 100-mesh sieve, mix evenly, and press into tablets In the hopper of the machine, adjust the pressure and filling amount, press into tablets, and get it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com