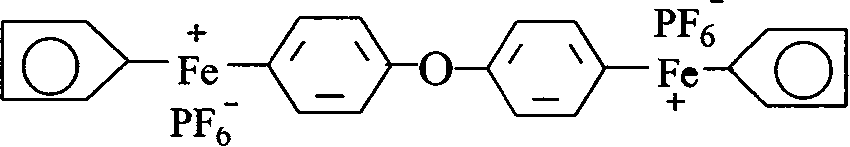
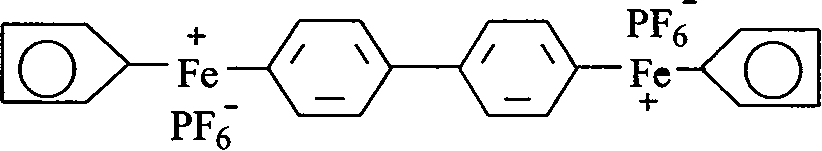
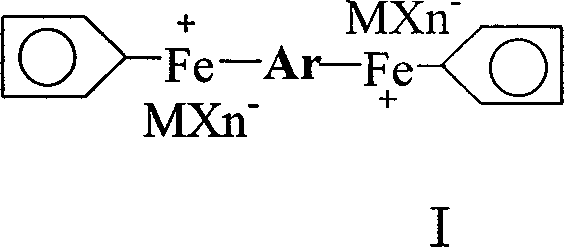Preparation of cation light initiator ferrocene arene salt and application thereof
A cationic and aromatic hydrocarbon technology, applied in the field of cationic photopolymerization initiators, can solve the problems of weak absorption, low photosensitizing activity, and cannot be a photosensitizer, etc., and achieves the effects of strong absorption and simple and convenient preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 prepares [two (cyclopentadiene-iron)-diphenyl ether] hexafluorophosphate
[0035] Put 0.15mol ferrocene, 7.83ml diphenyl ether, 0.3mol anhydrous AlCl into the three-necked flask 3 , 0.15 mol of Al powder and 200 ml of cyclohexane to stir and dissolve, then slowly raise the temperature to 80° C., and maintain the reflux state for 24 hours.
[0036] After the reaction solution is cooled, pour 5% methanol solution into it, stir and hydrolyze, filter, separate liquids, wash the organic phase with water, combine with the water phase, wash repeatedly with cyclohexane, and then add excess KPF 6 In the aqueous solution, a khaki precipitate precipitated out. The solid was dried and purified by column chromatography to obtain the product [bis(cyclopentadiene-iron)-diphenyl ether] hexafluorophosphate with a yield of 38.58%.
Embodiment 2
[0037] Embodiment 2 prepares [two (cyclopentadiene-iron)-carbazole] hexafluorophosphate
[0038] Put 0.135mol ferrocene, 0.045mol carbazole, 0.27mol anhydrous AlCl into the three-necked flask 3 , 0.135 mol of Al powder and 250 ml of cyclohexane to stir and dissolve, then slowly raise the temperature to 84° C., and maintain the reflux state for 18 hours.
[0039] After the reaction liquid is cooled, pour 10% methanol solution into it, stir and hydrolyze, filter, separate liquids, wash the organic phase with water, combine with the water phase, wash repeatedly with cyclohexane, and then add excess KPF 6 The aqueous solution has a yellow precipitate. The solid was dried and purified by column chromatography to obtain the product [bis(cyclopentadiene-iron)-carbazole] hexafluorophosphate with a yield of 66.7%.
Embodiment 3
[0040] Embodiment 3 prepares [two (cyclopentadiene-iron)-biphenyl] hexafluorophosphate
[0041] Put 0.1125mol ferrocene, 0.0375mol biphenyl, 0.225mol anhydrous AlCl into the three-necked flask 3 , 0.1125mol of Al powder and 210ml of decahydronaphthalene were stirred and dissolved, and then the temperature was slowly raised to 160° C., and the reflux state was maintained for 24 hours.
[0042] After the reaction liquid is cooled, pour 12% methanol solution into it, stir and hydrolyze, filter, separate liquids, wash the organic phase with water, combine with the water phase, wash repeatedly with cyclohexane, and then add excess KPF 6A pale yellow precipitate appeared in the aqueous solution. The solid was dried and purified by column chromatography to obtain the product [bis(cyclopentadiene-iron)-biphenyl]hexafluorophosphate with a yield of 49.12%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com